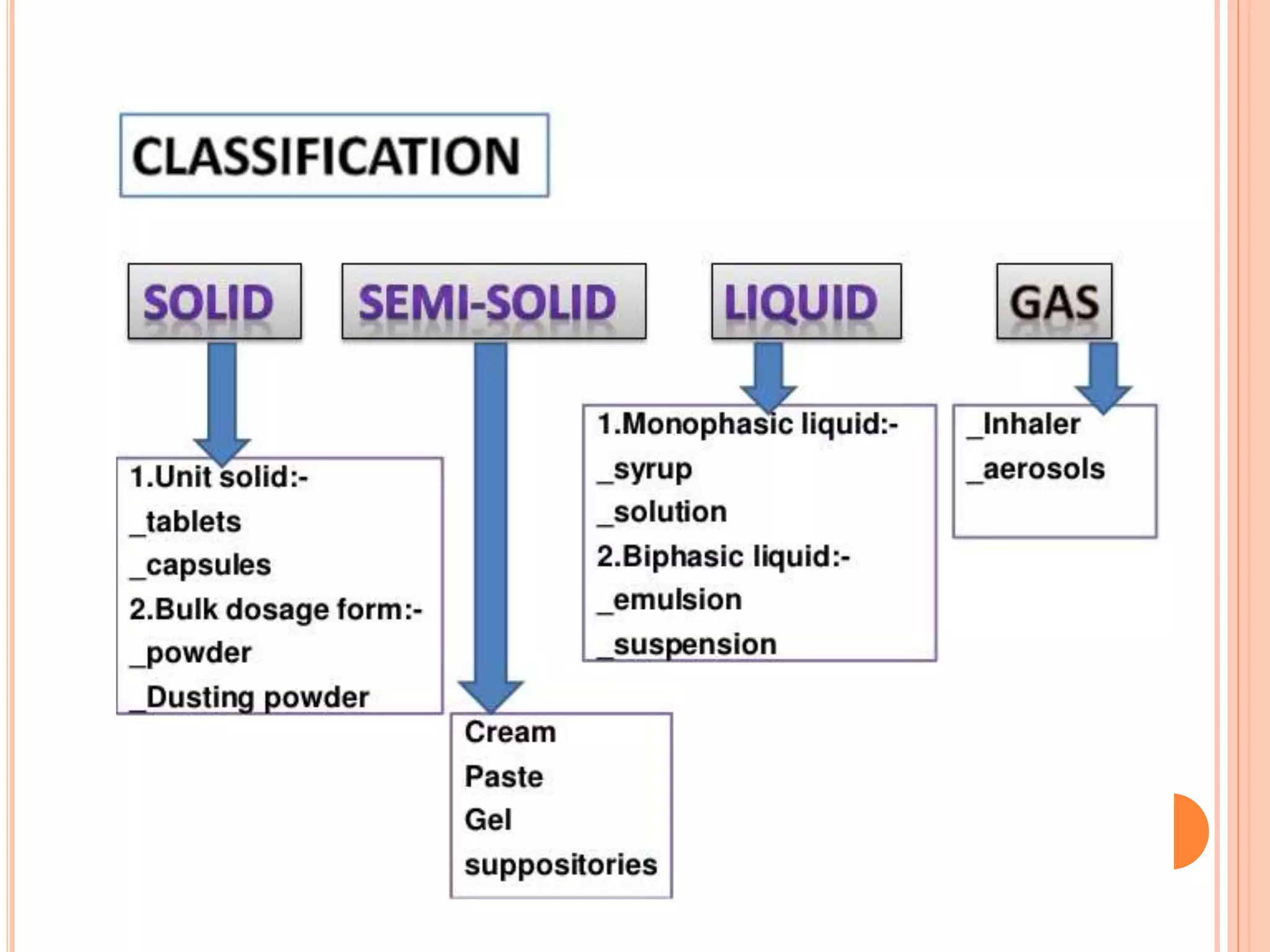
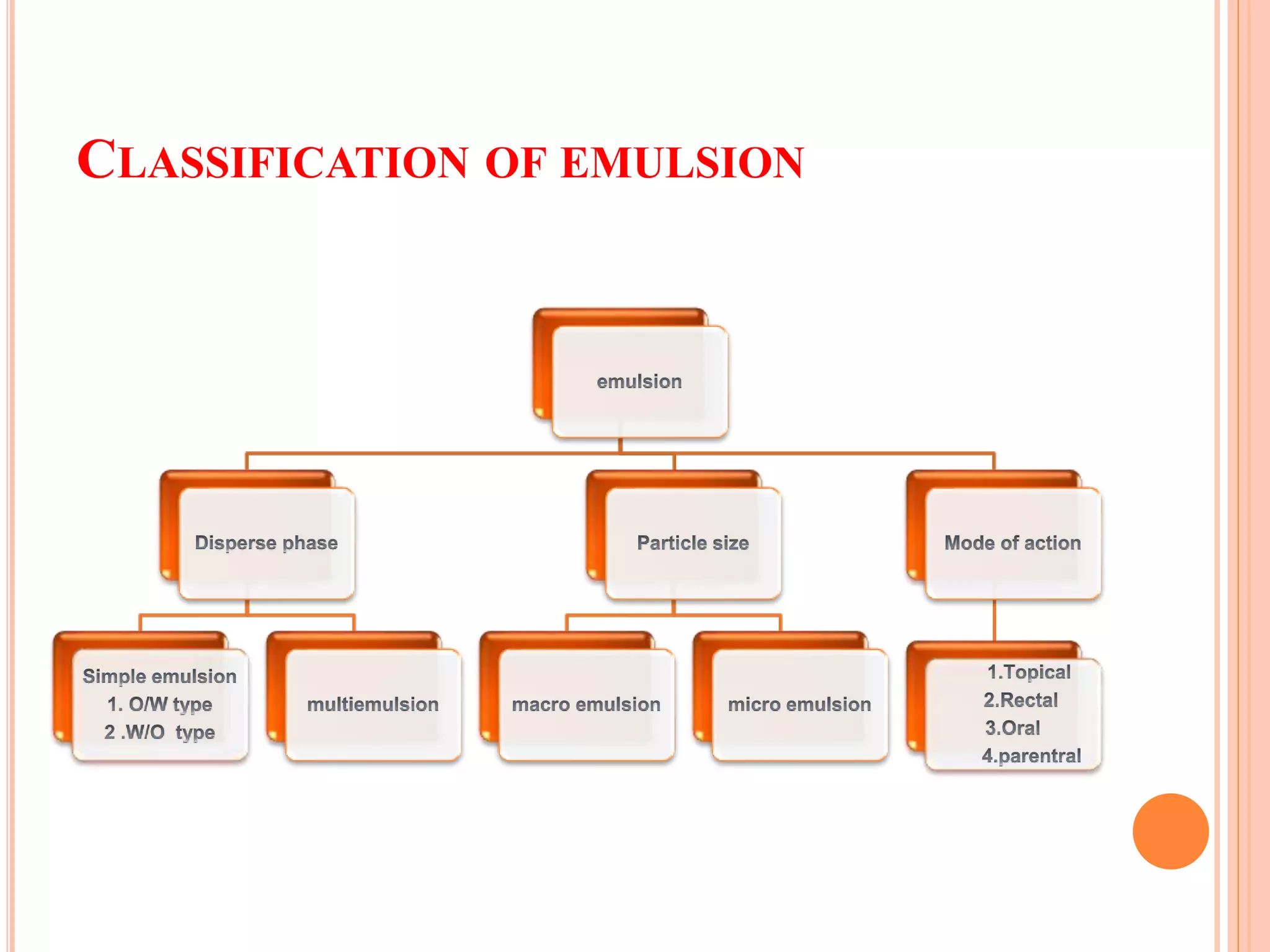
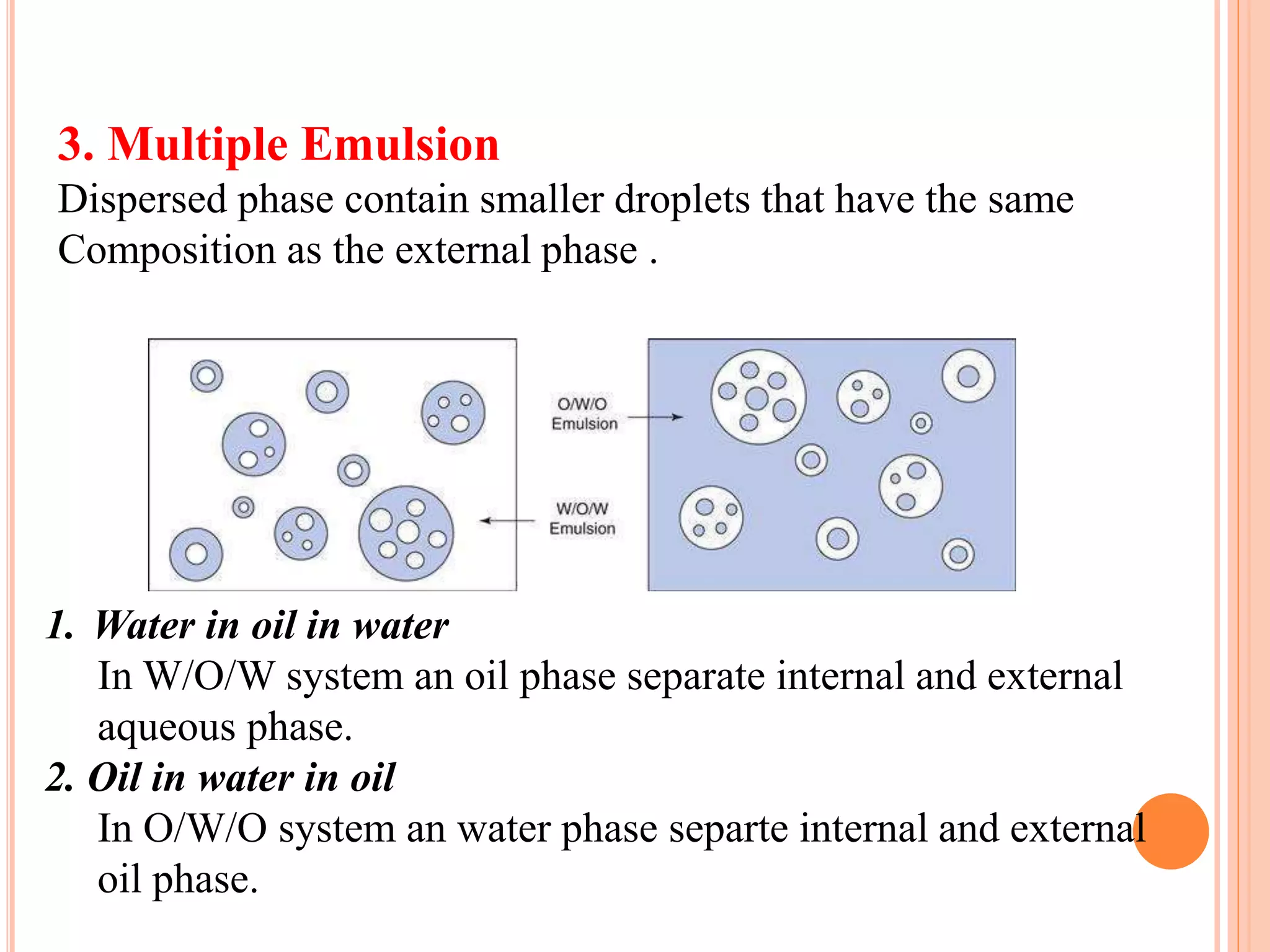
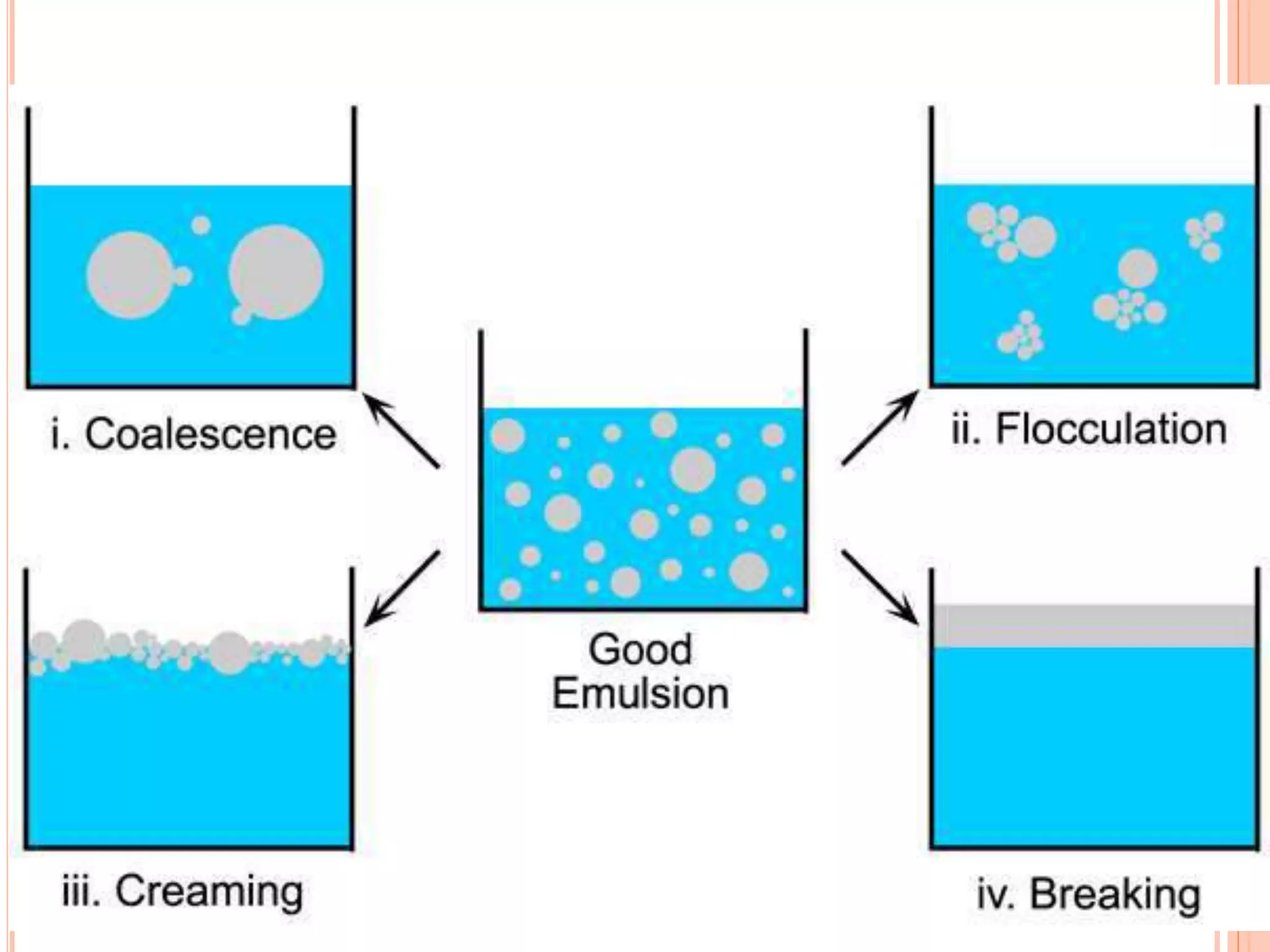
1) An emulsion is an unstable mixture of two immiscible liquids, where one liquid is dispersed as globules in the other liquid. Emulsions can be O/W (oil in water) or W/O (water in oil) types.
2) Pharmaceutical emulsions are used to deliver unpleasant tasting drugs, provide slow release of water-soluble drugs, and enhance absorption of oil-soluble drugs.
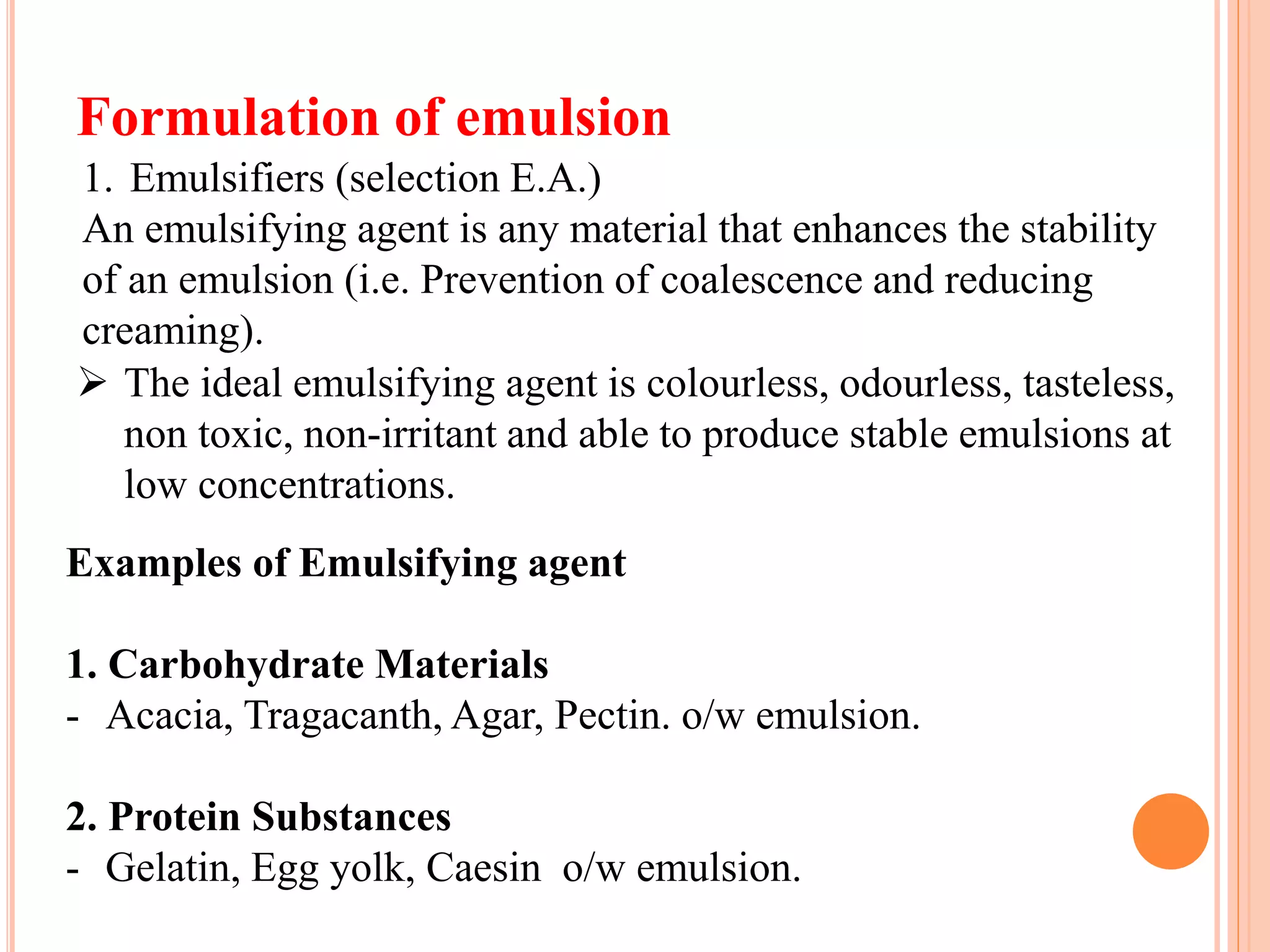
3) The key steps in formulating an emulsion are selecting an emulsifying agent based on its HLB value, adding preservatives and antioxidants, and using methods like trituration or the bottle method to prepare the emulsion.