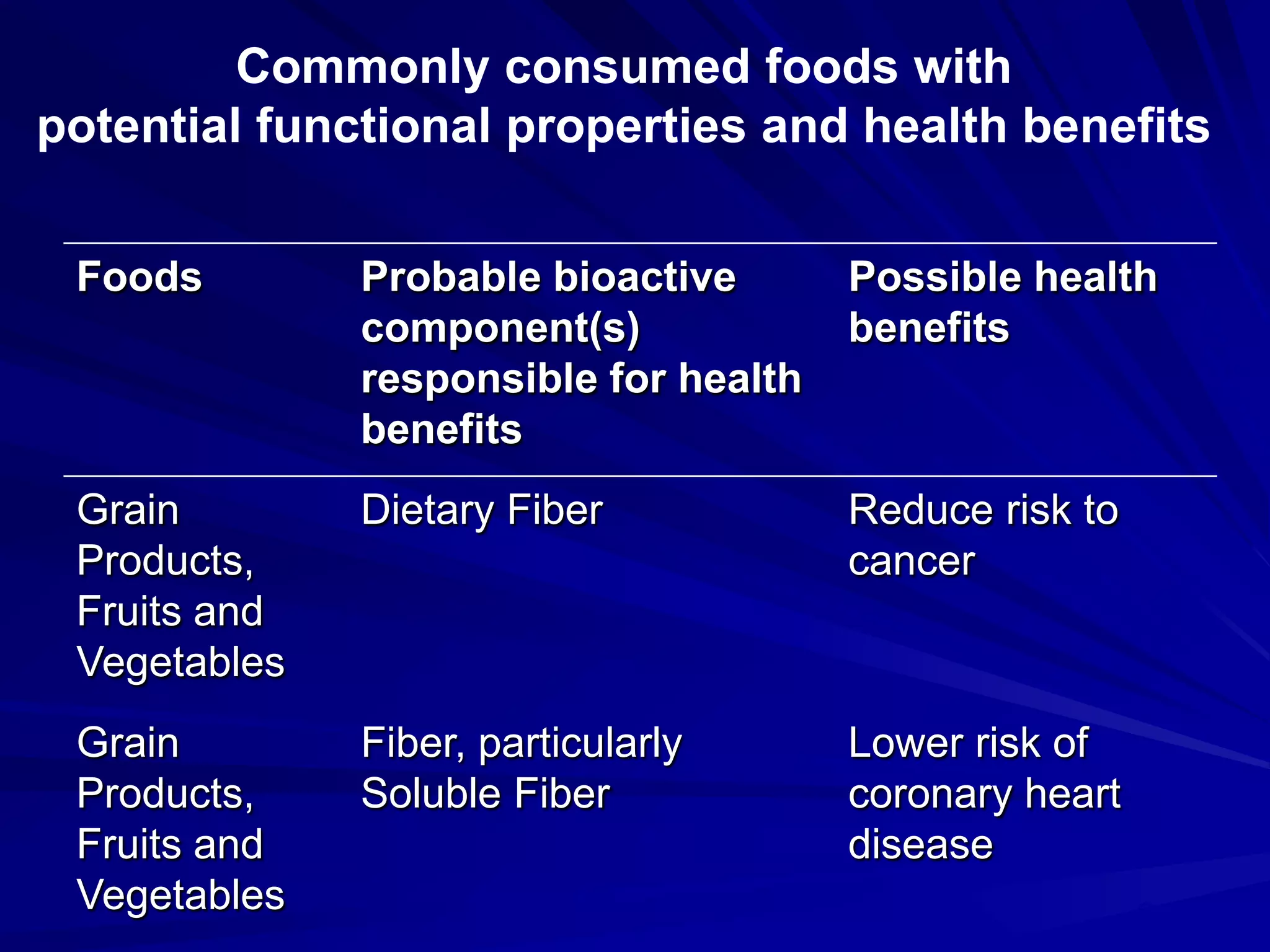
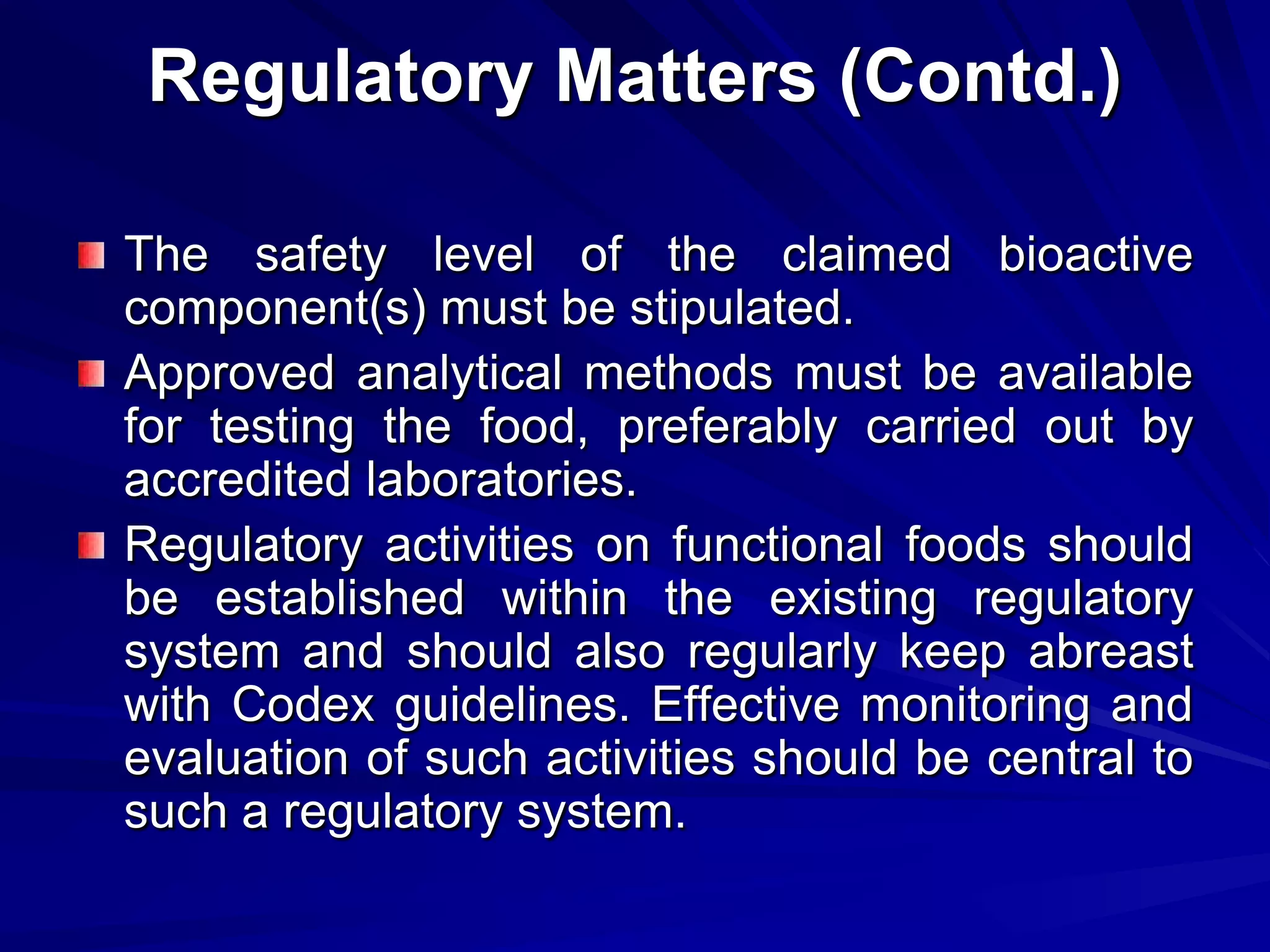
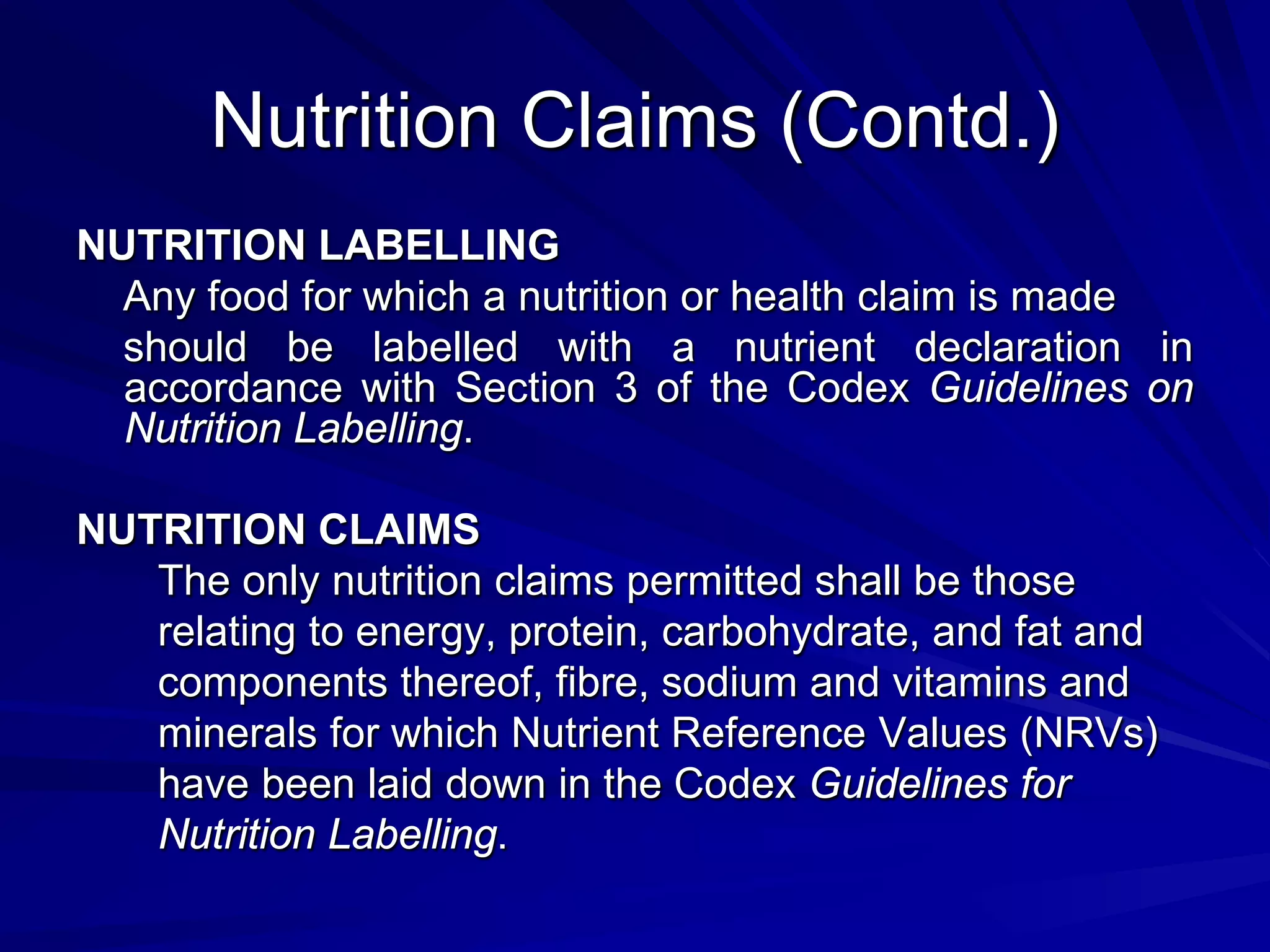
Codex provides guidance on nutrition and health claims for foods but does not have a definition or standards specifically for functional foods. [Codex recognizes the need for coordination on functional foods and held early discussions and workshops on topics related to safety, scientific evidence, and consumer issues.] Codex guidelines indicate that only nutrition claims relating to energy, major nutrients, fiber, sodium and vitamins/minerals are permitted, and set standards for nutrient content claims and reduction of disease risk claims. National authorities are responsible for regulating functional foods in their markets based on Codex guidance.