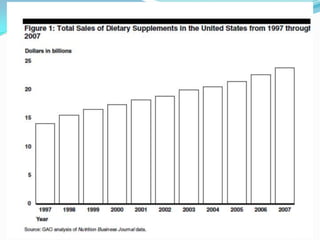
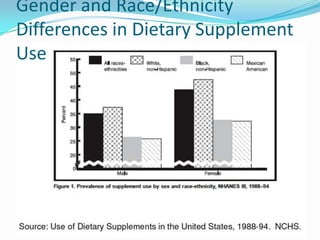
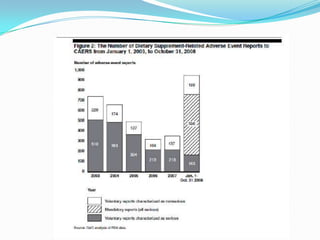
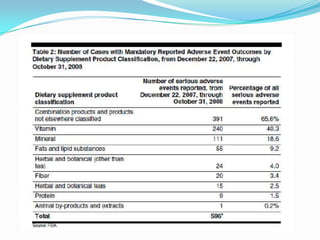
The document discusses the role of government in regulating the dietary supplement market. It notes that the market is large, with over $23 billion in annual sales and over 29,000 products. However, the FDA has limited regulatory authority over supplements due to DSHEA. This creates an information asymmetry between consumers and manufacturers. The document examines issues of safety, effectiveness and quality assurance in the supplement industry. It recommends that the FDA be granted more authority to issue guidance and ensure consumer understanding of supplements.