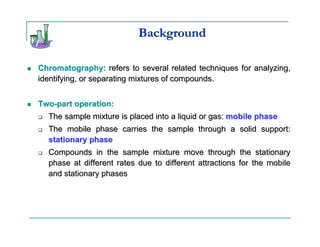
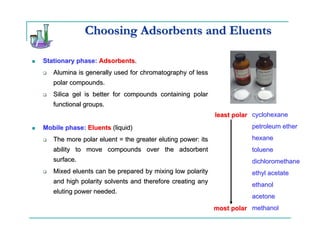
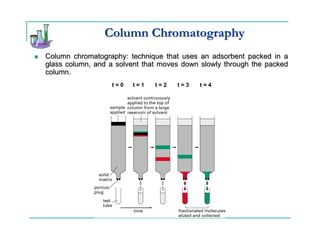
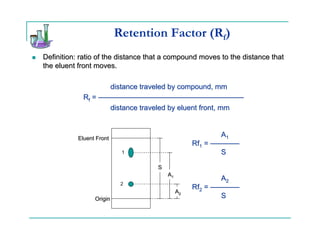
This document provides background information on thin-layer chromatography (TLC) and column chromatography. It describes the basic two-part operation involving a mobile phase that carries samples through a stationary phase. The document discusses factors that influence separation, such as choosing adsorbents and eluents. It then describes how to perform TLC and column chromatography experiments, including preparing plates/columns, developing separations, and analyzing results. Procedures are outlined for identifying unknown compounds and disposing of waste.