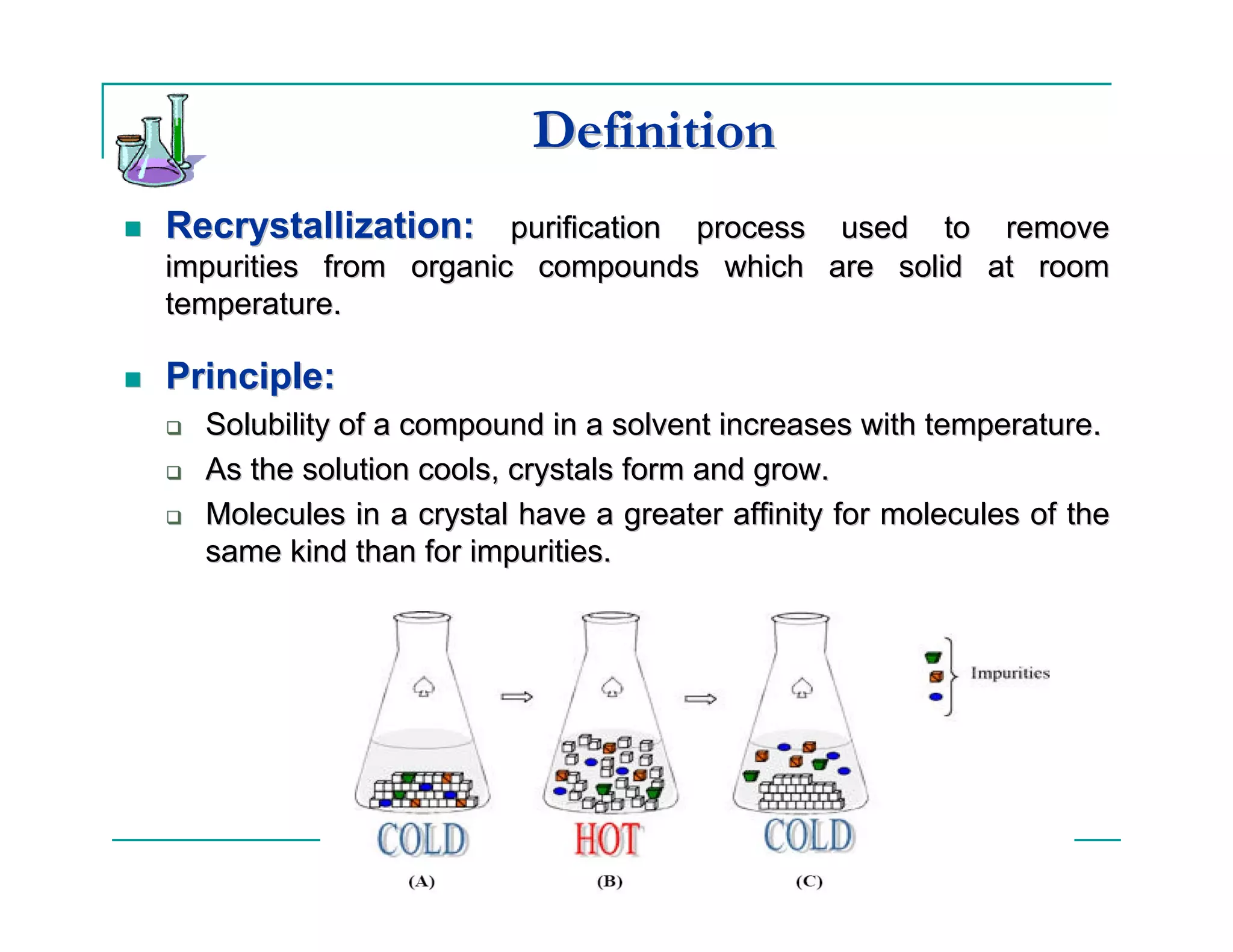
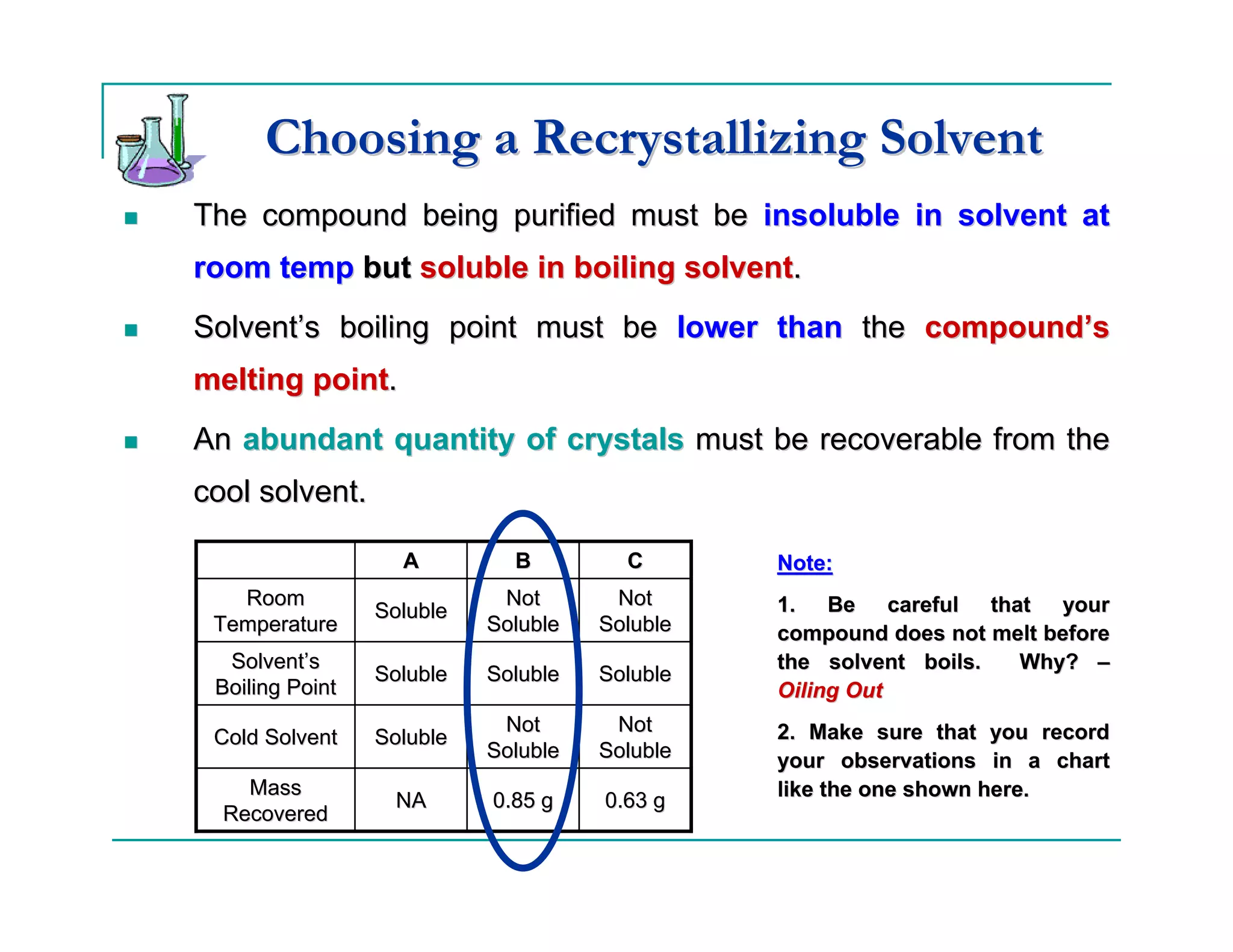
This document describes the process of recrystallization used to purify organic compounds. Recrystallization involves dissolving the impure compound in a hot solvent, removing any insoluble impurities through filtration, inducing crystal formation as the solution cools, collecting the purified crystals, and determining the percent recovery. The key steps are choosing an appropriate solvent based on the compound's solubility properties, dissolving and filtering the heated solution, slowly cooling the solution to form crystals, washing and drying the crystals, and calculating the percent recovery by mass and melting point.