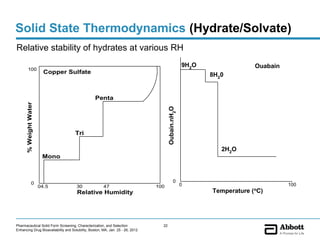
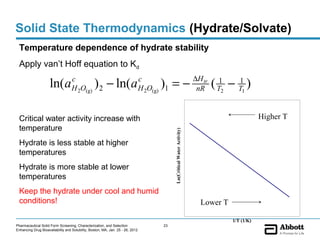
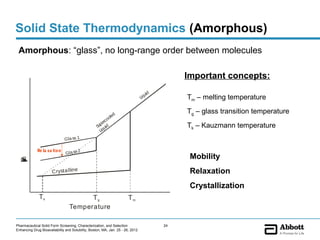
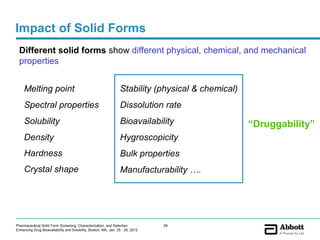
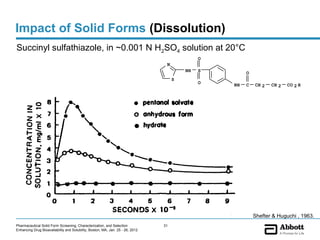
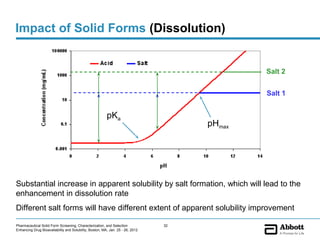
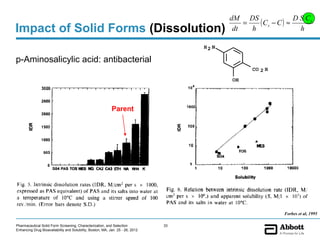
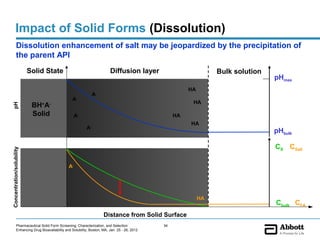
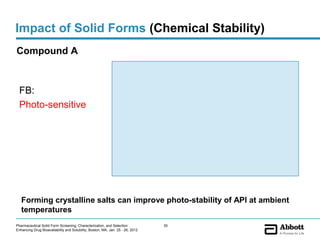
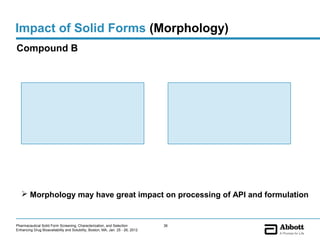
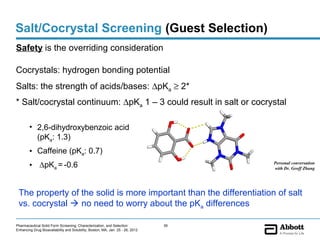
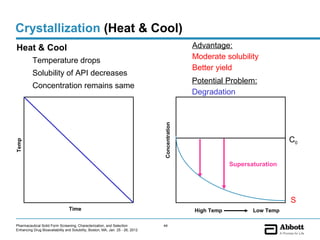
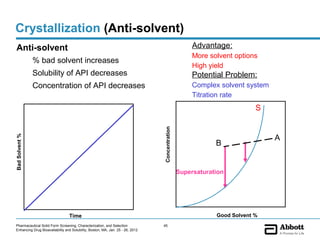
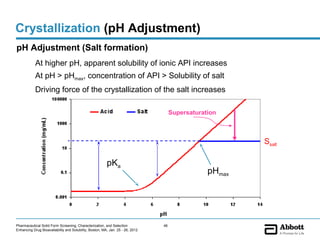
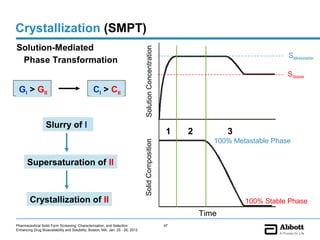
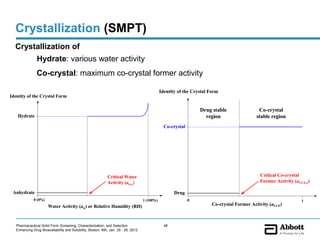
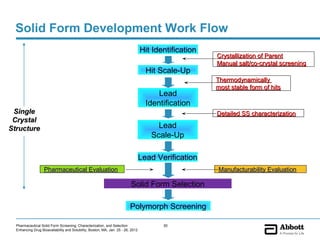
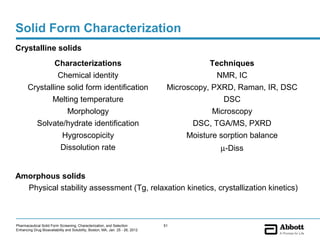
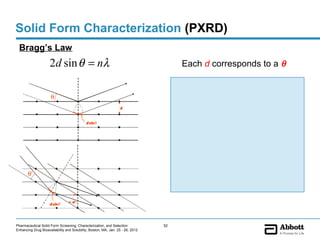
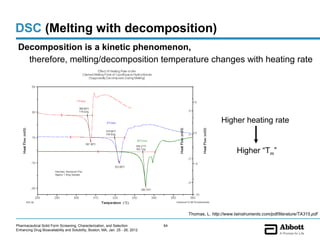
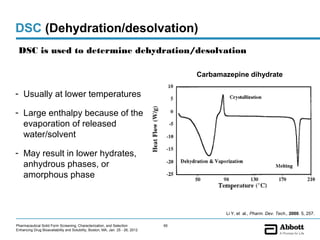
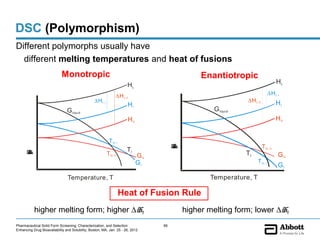
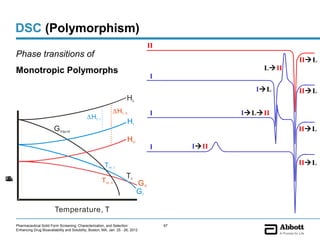
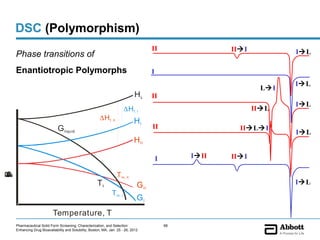
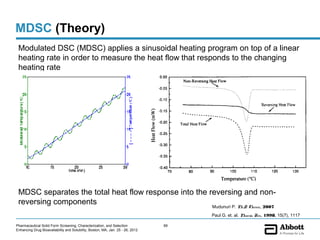
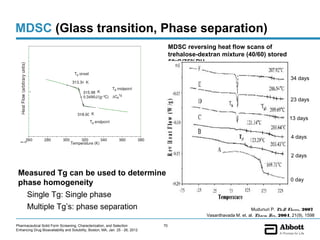
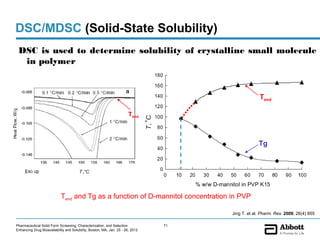
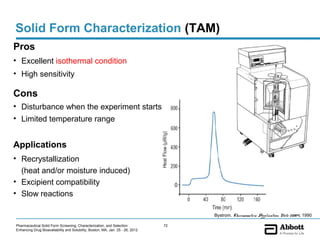
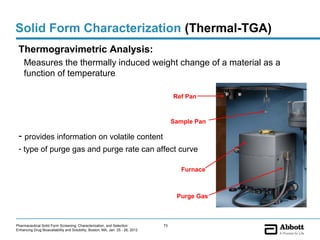
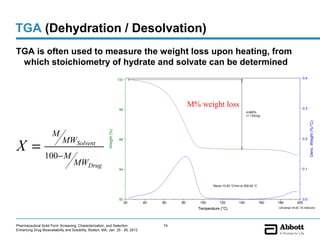
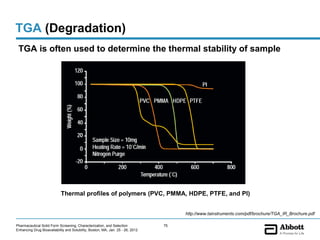
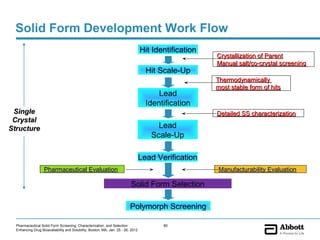
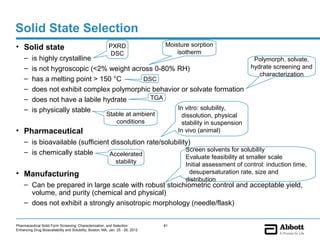
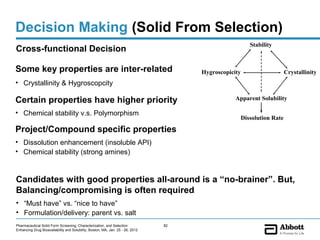
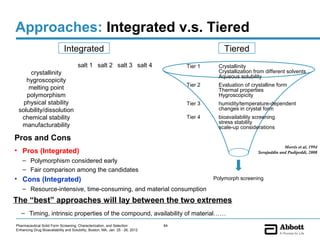
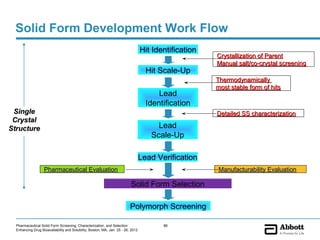
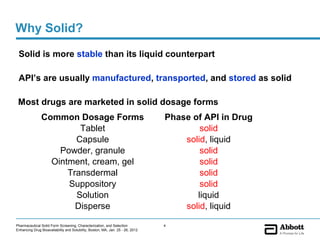
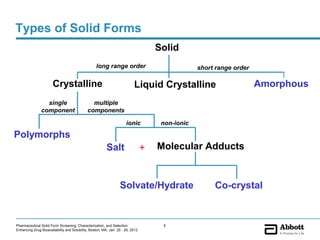
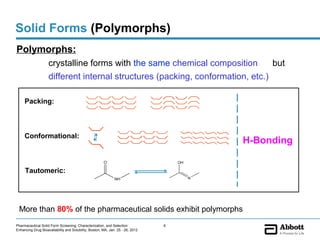
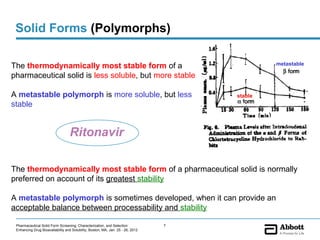
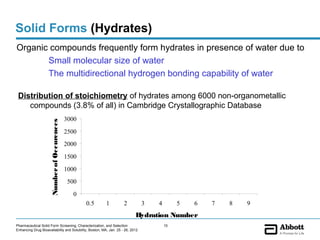
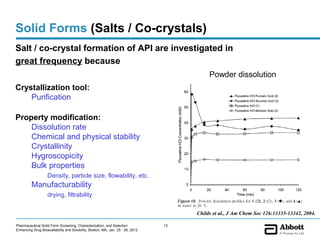
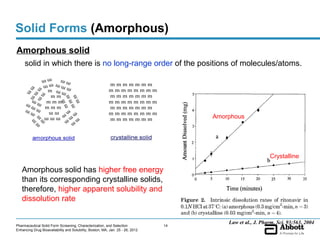
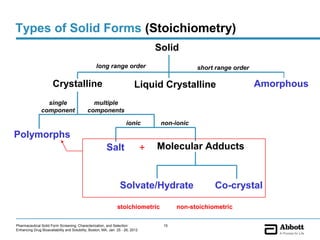
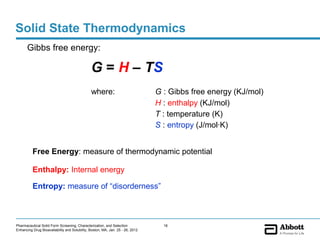
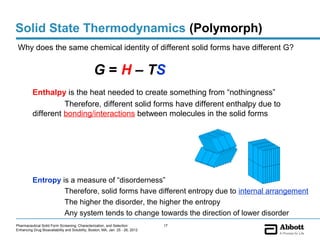
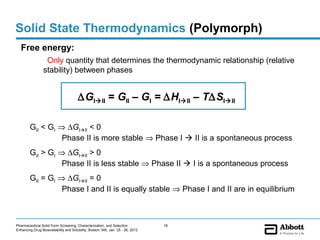
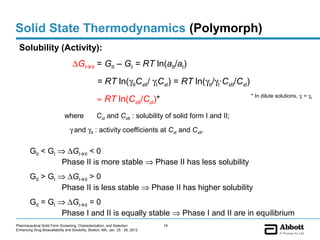
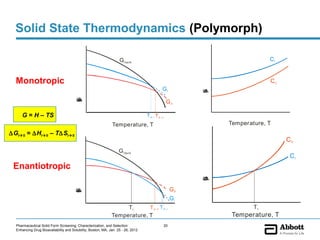
The document discusses different types of solid forms that active pharmaceutical ingredients can take, including polymorphs, solvates, hydrates, salts, co-crystals, and the amorphous form. It notes that over 80% of pharmaceutical solids exhibit polymorphism. The thermodynamically most stable polymorph is generally preferred for stability reasons, though a metastable polymorph may be developed to provide a balance between processability and stability. Hydrates and solvates are discussed, with hydrates being the most common type of solvate. Salt and co-crystal formation can impact properties like dissolution and stability. The amorphous form lacks long-range order.


![Solid State Thermodynamics (Hydrate/Solvate)
The equilibrium between the anhydrous and hydrate forms of a drug D
can be represented as
K , p ∆H
D ⋅ nH 2O( solid ) ← → D( solid ) + nH 2O( gas )
d t,
tr
[aD(s) ][aH 2O(g) ]n
c
p
Therefore Kd = = [aH 2O(g) ]n = [ pt ]n = [ RH ]n
c
[aD⋅nH 2O(s) ] s
aH 2O(g) > aH 2O(g) or p > pt , hydrate is more stable
c
aH 2O(g) < aH 2O(g) or p < pt , anhydrate is more stable
c
aH 2O(g) = aH 2O(g) or p = pt , anhydrate and hydrate are
c
equally stable
* Solvates are treated similarly
Pharmaceutical Solid Form Screening, Characterization, and Selection 21
Enhancing Drug Bioavailability and Solubility, Boston, MA, Jan. 25 - 26, 2012](https://image.slidesharecdn.com/solubility-boston-2012-published-121128154818-phpapp01/85/Solubility-boston-2012-published-21-320.jpg)