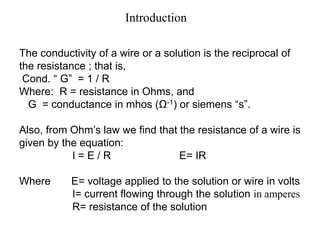
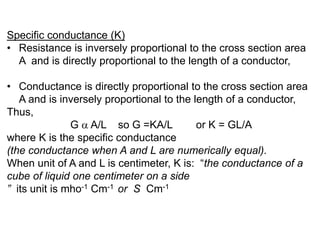
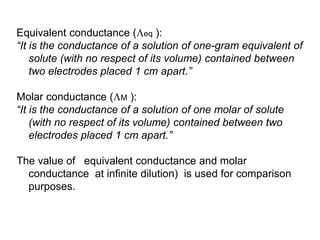
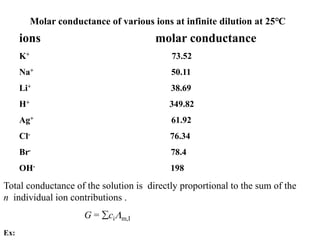
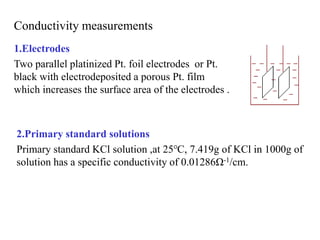
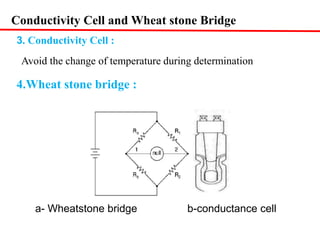
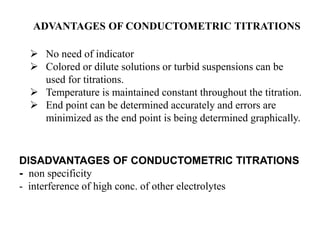
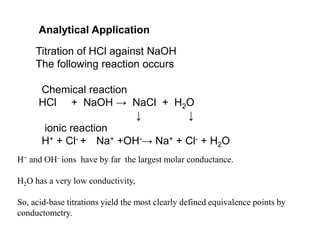
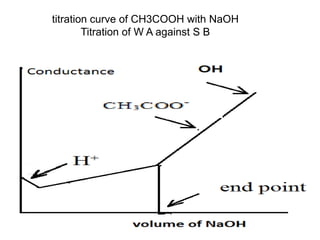
Conductometry is a simple electroanalytical technique that measures the ability of a solution to conduct electricity via ion movement. Conductivity depends on the number and mobility of ions present. Factors like ion size/hydration, temperature, and viscosity affect ion mobility. Specific conductance and molar conductance are used to compare conductivities between solutions. Conductometric titrations can determine endpoints accurately without indicators and are used to analyze solubility, equilibria, salinity, and more. Titration curves are plotted from changes in conductivity as a function of titrant added.