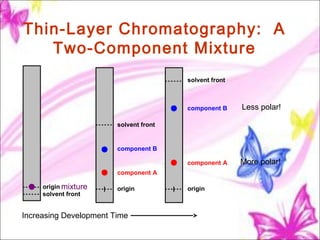
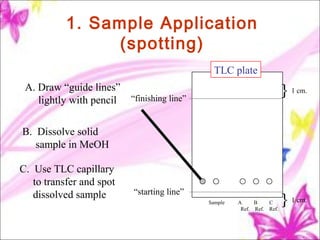
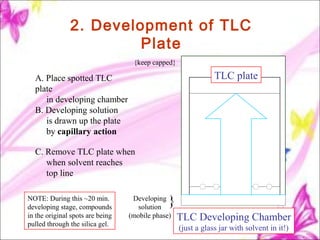
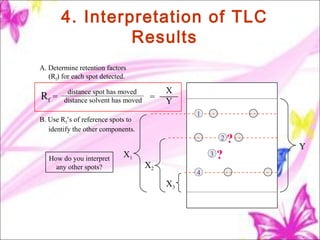
The document discusses planar chromatography, including its mechanisms, classifications, and detailed procedures for techniques like thin-layer chromatography (TLC) and paper chromatography. It outlines the stages of TLC, such as sample application, development, visualization, and interpretation, as well as applications for separating various substances. Overall, it serves as a comprehensive guide to understanding and implementing planar chromatography methods in pharmaceutical analysis.