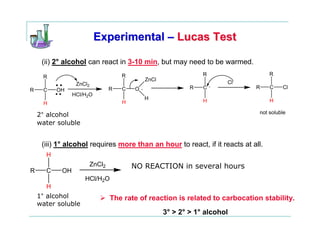
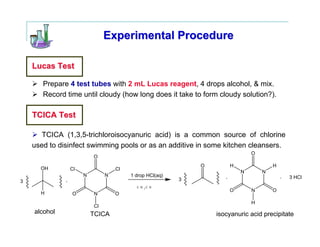
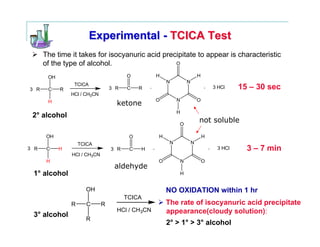
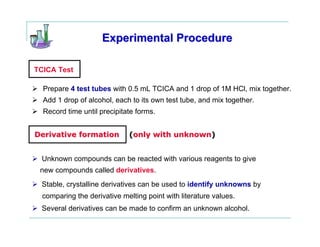
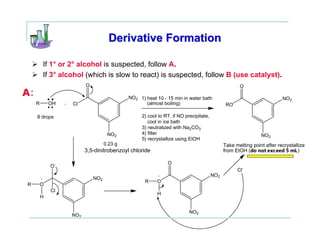
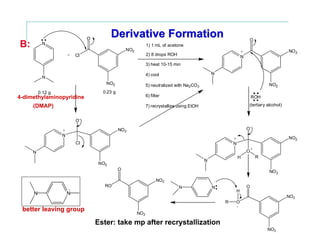
This document provides instructions for classifying an unknown alcohol through a series of classification tests. It describes performing the Lucas test, TCICA test, and forming derivatives of the unknown compound to aid in identification. The Lucas test distinguishes between primary, secondary and tertiary alcohols based on reaction rates. The TCICA test also distinguishes alcohol types by the time taken for a precipitate to form. Derivatives can be made and have melting points compared to literature values to identify the unknown alcohol. Proper experimental procedure and waste disposal are emphasized.