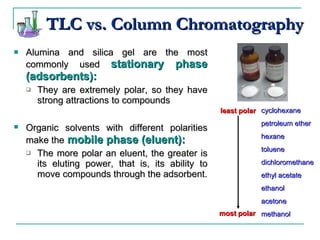
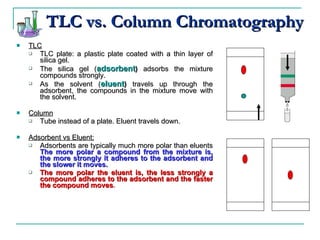
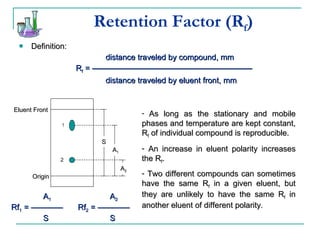
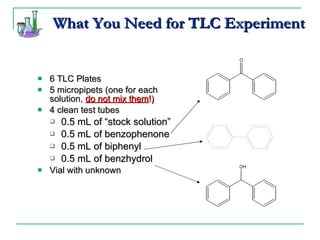
This document provides background on thin-layer chromatography (TLC) and column chromatography techniques. It describes the basic two-part operation involving a mobile phase that carries samples through a stationary phase. Common stationary phases like silica gel and alumina are discussed. The document also summarizes key steps for performing TLC, including spotting samples, developing the plate, and visualizing results. Procedures for running a column chromatography experiment to separate ferrocene and acetylferrocene are also outlined.