Complexes are assemblies of a central metal ion bonded to surrounding ligands. Common ligands include NH3, CN-, OH-, and CO. Transition metals can form complexes with varying coordination numbers and oxidation states. Complex ions undergo ligand substitution and acid-base decomposition reactions in solution.

![Complexes: assemblies of a central metal ion bonded to a group of surroundings molecules or ions. Ex. [Ag(NH 3 ) 2 ] + Complex Ion: If the complex carries a charge Coordination Compounds: Compounds that contain complexes](https://image.slidesharecdn.com/jibt10-110606211100-phpapp02/85/Chemistry-JIB-T10-Transition-Metals-2-320.jpg)



![They are either polar or anions Because ligands have lone pairs, they can function as Lewis bases This makes the metal a Lewis acid Ligand coordiante to the metal Ex. [Cu(NH 3 ) 4 ]SO 4 [Cu(NH 3 ) 4 ] 2+ CATION SO 4 2- ANION](https://image.slidesharecdn.com/jibt10-110606211100-phpapp02/85/Chemistry-JIB-T10-Transition-Metals-7-320.jpg)
 2 [Ru(NH 3 ) 5 (H 2 O)]Cl 2 [Cr(NH 3 ) 6 ](NO 3 ) 3 K 4 [Fe(CN) 6 ]](https://image.slidesharecdn.com/jibt10-110606211100-phpapp02/85/Chemistry-JIB-T10-Transition-Metals-8-320.jpg)
![The atom of the ligand bound directly to the metal is called the donor atom Ex. [Ag(NH 3 ) 2 ] + N is the donor atom The coordination number (the number of donor atoms attached to the metal) in the above example is 2. Dependent on the number of donor atoms present, ligands are classified as monodentate, bidentate, or polydentate Ex. Monodentate: H 2 O Bidentate: ethylenediamine: H 2 N-CH 2 –CH 2 –NH 2 Bidentate and polydentate ligands are called chelating agents : They hold the metal atom like a claw.](https://image.slidesharecdn.com/jibt10-110606211100-phpapp02/85/Chemistry-JIB-T10-Transition-Metals-9-320.jpg)
![In naming salts, the name of the cation is given before the name of the anion. Ex. In [Co(NH 3 ) 5 Cl]Cl 2 , we name the [Co(NH 3 ) 5 Cl] 2+ and then Cl - . Within a complex ion or molecule, the ligands are named before the metal. Prefixes that give the number of ligands are not considered part of the ligand name in determining alphabetical order. In [Co(NH 3 ) 5 Cl] 2+ ion, the ammonia ligands are named first, then the chloride, then the metal: Pentaaminechlorocobalt (III)](https://image.slidesharecdn.com/jibt10-110606211100-phpapp02/85/Chemistry-JIB-T10-Transition-Metals-10-320.jpg)
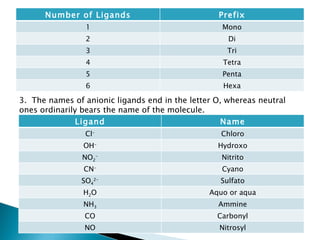
![A Greek prefix is used to indicate the number of each kind of ligand when more than one is present. If the name of the ligand itself contains a Greek prefix, such as mono- or di-, the name of the ligand is enclosed in parentheses and alternate prefixes are used, Bis-, Tris-, tetrakis-,hexakis Ex. [Co(en) 3 ]Cl 3 tris(ethylenediamine)cobalt (III) chloride If the complex is an anion, its name ends in –ate. Ex. K 4 [Fe(CN) 6 ] hexocyanoferrate (II) ion Others: Copper: Cuprate Silver: Argenate](https://image.slidesharecdn.com/jibt10-110606211100-phpapp02/85/Chemistry-JIB-T10-Transition-Metals-12-320.jpg)
![The oxidation number of the metal is given in parentheses in Roman numerals following the name of the metal. Try naming these: [Cr(H 2 O) 4 Cl 2 ]Cl [Co(NH 3 ) 4 (H 2 O)CN]Cl 2 Na[Al(OH) 4 ] K 2 [Ni(CN) 4 ] (NH 4 ) 2 [CuBr 4 ] [Ni(H 2 O) 6 ]SO 4 [CoCl 3 (NH 3 ) 3 ] 1- [Co(NH 3 ) 6 ]Cl 3 K 3 [Fe(CN) 6 ] K 4 [Fe(CN) 6 ]](https://image.slidesharecdn.com/jibt10-110606211100-phpapp02/85/Chemistry-JIB-T10-Transition-Metals-13-320.jpg)
![☺ Central ion is first ☺ Ligands second ☺ Neutral molecules first ☺ Negative ions second ☺ Neutral molecules and polyatomic ions are always surrounded by (parentheses) , even if only 1 present ☺ Entire complex ion formula surrounded by [brackets]](https://image.slidesharecdn.com/jibt10-110606211100-phpapp02/85/Chemistry-JIB-T10-Transition-Metals-14-320.jpg)
![Write the formula for the diamminepalladium(II) ion ☺ Central ion – palladium (Pd) ☺ Ligands – NH 3 (2) ☺ Written in parentheses ☺ Neutral charge ☺ Brackets around entire formula ☺ Calculate the net charge [Pd(NH 3 ) 2 ] 2+](https://image.slidesharecdn.com/jibt10-110606211100-phpapp02/85/Chemistry-JIB-T10-Transition-Metals-15-320.jpg)
![Write the formula for the carbonylpentacyanoferrate(II) ion ☺ Central ion – iron (Fe) ☺ Ligands ☺ CN (5) 1- charge Written in parentheses ☺ CO (1) Neutral Written in parentheses ☺ Brackets around entire formula ☺ Calculate the net charge [Fe(CO)(CN) 5 ] 3-](https://image.slidesharecdn.com/jibt10-110606211100-phpapp02/85/Chemistry-JIB-T10-Transition-Metals-16-320.jpg)
![Tetraammineplatinum(II) ion [Pt(NH 3 ) 4 ] 2+ Tetraiodoaurate(III) ion [AuI 4 ] - Pentacarbonylnitrosyliron(II) ion [Fe(CO) 5 (NO)] 2+](https://image.slidesharecdn.com/jibt10-110606211100-phpapp02/85/Chemistry-JIB-T10-Transition-Metals-17-320.jpg)
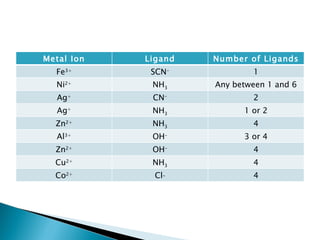
![Ligand Exchange Reactions of Transition Metals (and Al 3+ ) Complex ions undergo ligand substitution reactions in solutions Ex. [Ni(H 2 O) 6 ] 2+ + NH 3 -> [Ni(NH 3 ) 6 ] 2+ + H 2 O or Ni 2+ + NH 3 -> [Ni(NH 3 ) 6 ] 2+ How do you know the number of ligands that are in the product? Hint: Often, not all the time!!, the number of ligands is twice the cation charge. Also, helpful to know the following table](https://image.slidesharecdn.com/jibt10-110606211100-phpapp02/85/Chemistry-JIB-T10-Transition-Metals-18-320.jpg)
![Decomposition of complex by acid-base neutralization Complexes containing NH 3 can be broken down by acid base decomposition by adding an acid Ex. [Cu(NH 3 ) 4 ] 2+ + H + -> Cu 2+ + NH 4 + The NH 3 acts as a base (accepts H + ions to form NH 4 + ). The rest of the complex is broken down. Go over sheet from the “Ultimate Chemical Equation Handbook.” Responsible for all the reactions listed in the transition metal section.](https://image.slidesharecdn.com/jibt10-110606211100-phpapp02/85/Chemistry-JIB-T10-Transition-Metals-20-320.jpg)