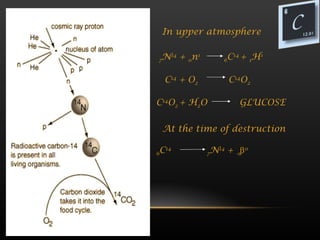
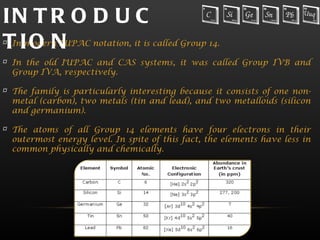
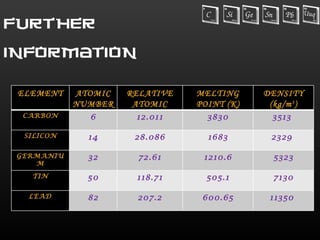
The document provides information about the elements in Group 14 of the periodic table. It begins by introducing the group and listing the elements carbon, silicon, germanium, tin, and lead. It then provides details about each element, including their physical properties, oxidation states, occurrence in nature, and important uses. The document discusses topics like allotropes of carbon, silicon semiconductor applications, germanium use in electronics, tin uses in alloys and solder, and properties of lead like its low melting point.









![c a rbo
n
In diamond, the atoms are arranged tetrahedrally in a vast
continuous array.In graphite, hexagonal rings are joined
together to form sheets, and the sheets lie one on top of the other.
In 1961 the International Union of Pure and Applied Chemistry
[IUPAC] adopted the isotope carbon-12 as the basis for relative
atomic masses.
Carbon atoms in diamond Carbon atoms in graphite](https://image.slidesharecdn.com/pblockgroup14-120314102007-phpapp02/85/P-block-group-14-14-320.jpg)