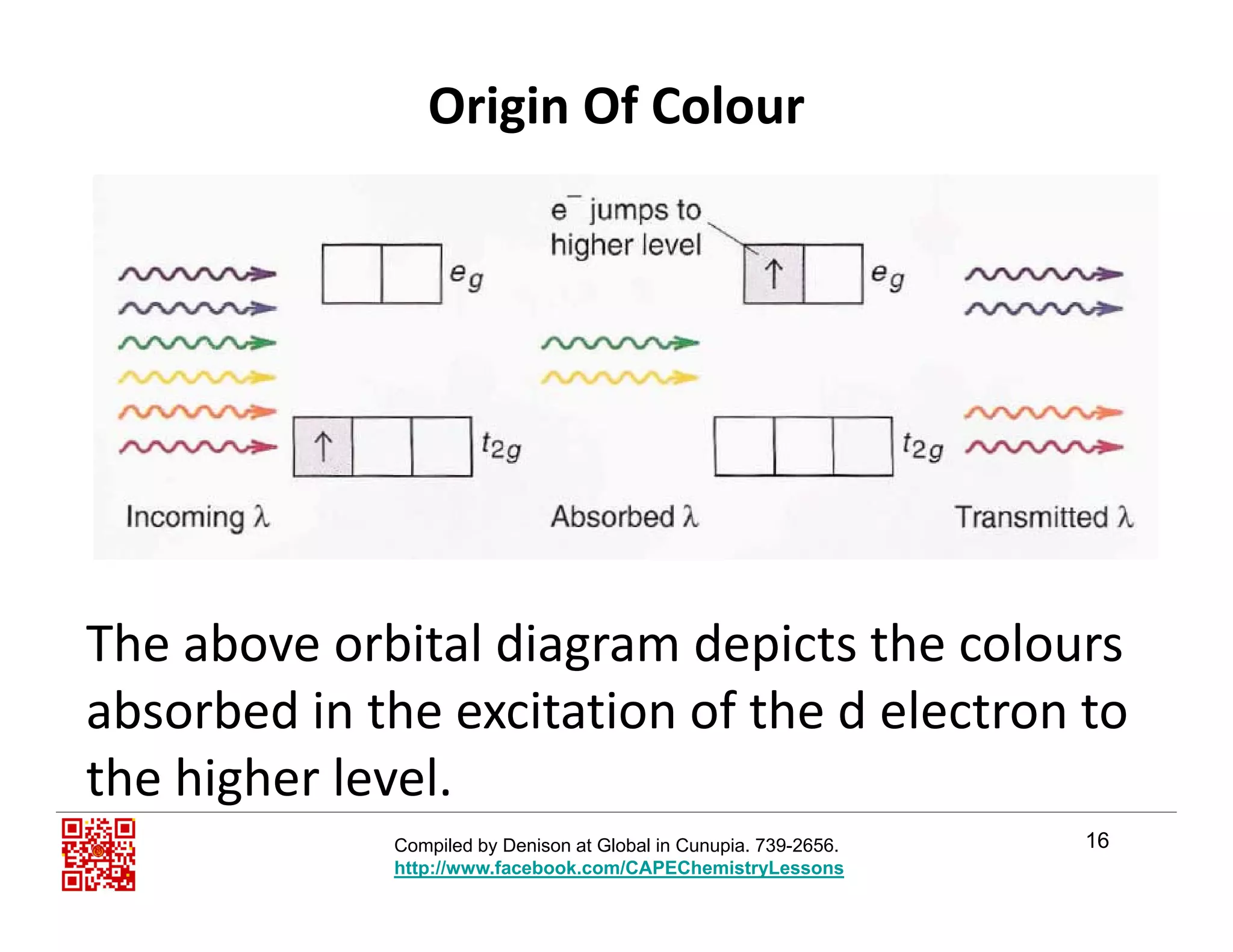
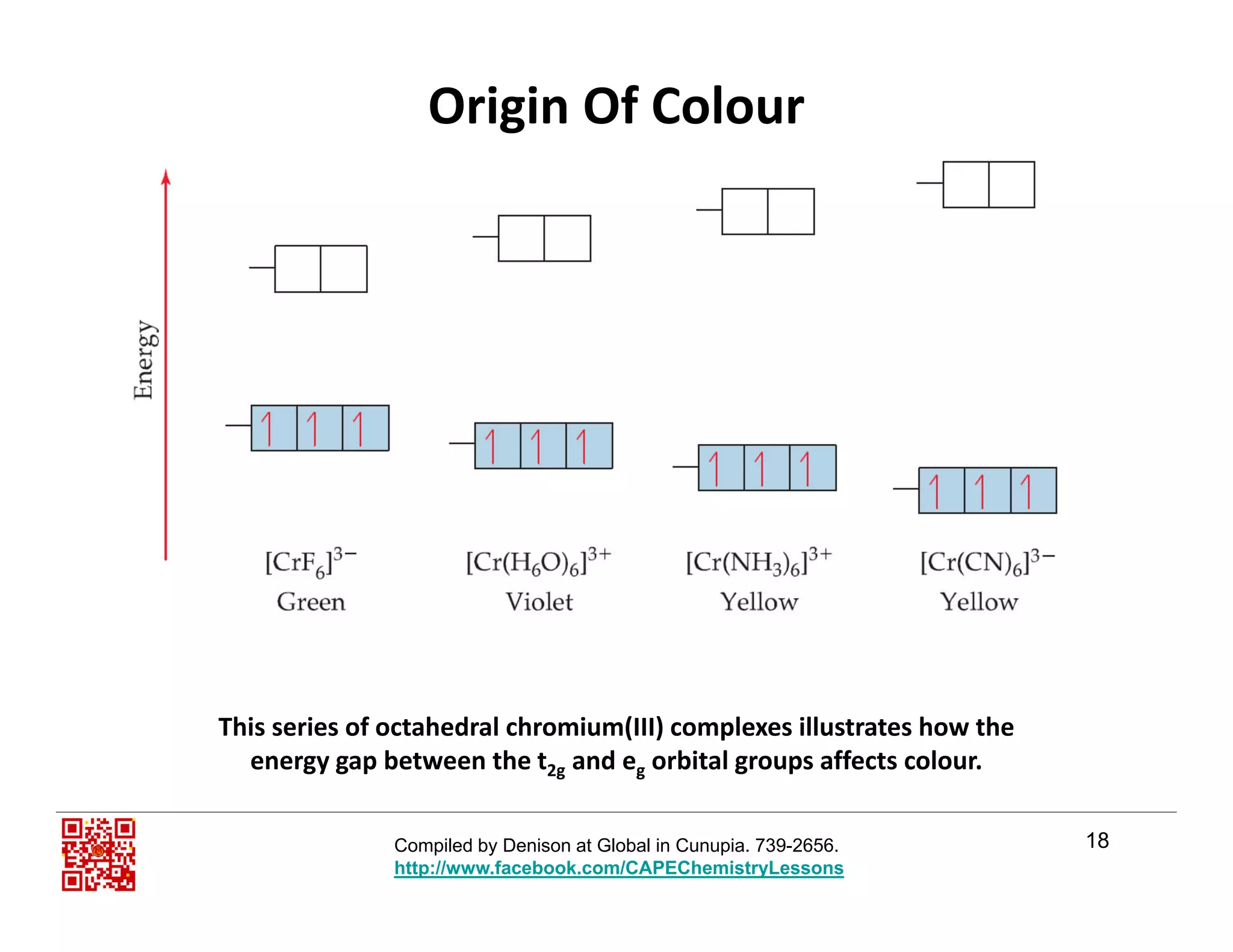
The document outlines the origin of color in transition metal complexes, focusing on crystal field theory and ligand field theory, which explain how the arrangement and energy splitting of d orbitals affect color perception. It highlights six key points necessary for answering related questions in CAPE exams, emphasizing the significance of partially filled d orbitals, ligand interaction, and the energy gap between orbitals. Additionally, it discusses how ligands influence the color of transition metal ions and the complexity of the overall color theory.













![Origin Of Colour
1. For a given ligand, the colour depends
th id ti t t f th t l ion the oxidation state of the metal ion.
A solution of [V(H2O)6]2+ ion is violet, and
a solution of [V(H2O)6]3+ ion is yellow.
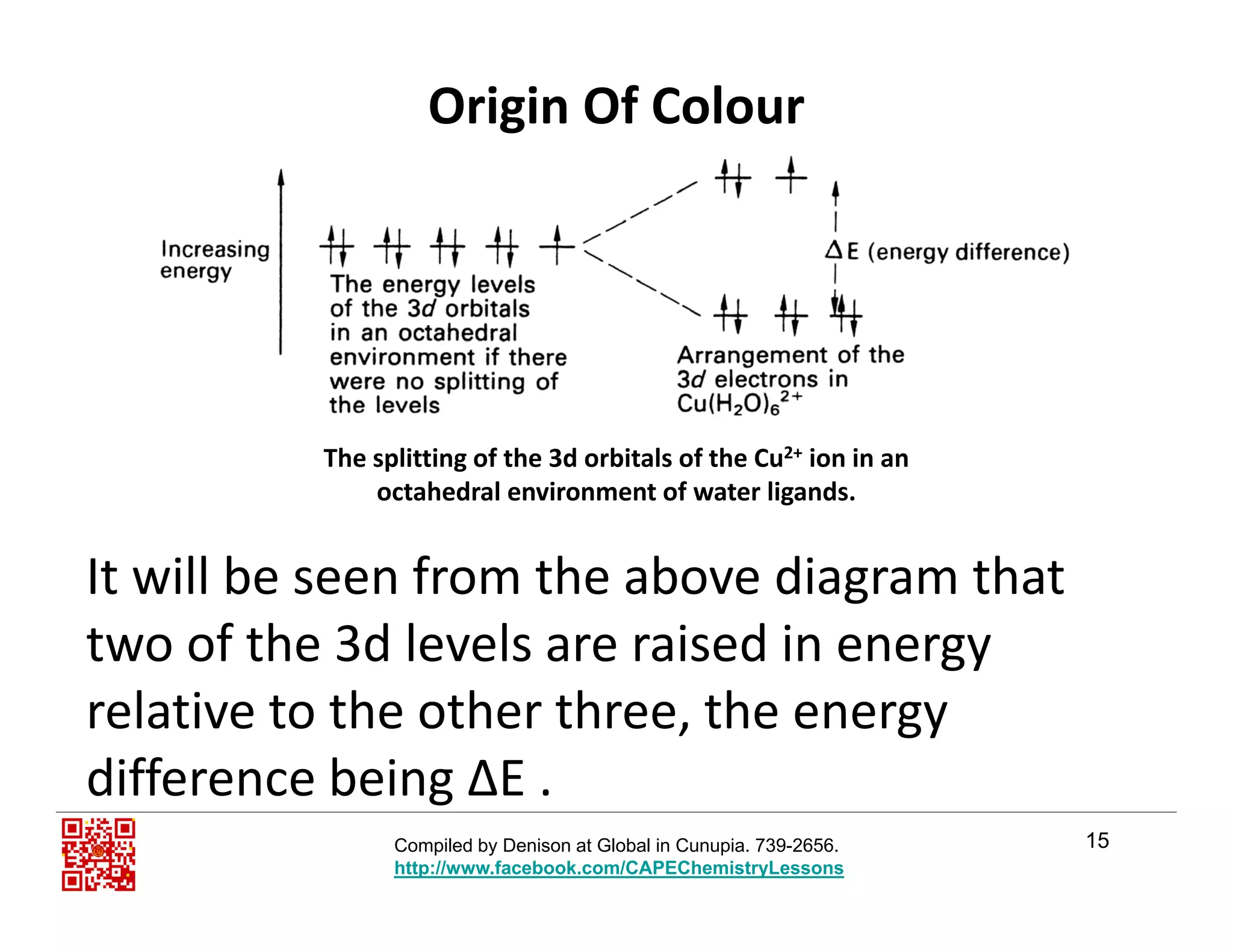
Solutions of [V(H2O)6]2+ (left) and[V(H2O)6]3+ (right) ions have different colours.
20
2 6 2 6
Compiled by Denison at Global in Cunupia. 739-2656.
http://www.facebook.com/CAPEChemistryLessons](https://image.slidesharecdn.com/transitionelements-originofcolour-130506210548-phpapp02/75/Transition-Elements-Origin-Of-Colour-20-2048.jpg)
![Origin Of Colour
2. For a given metal ion, the colour
d d th li d E i ldepends on the ligand. Even a single
ligand substitution can have a major
effect on the wavelengths absorbed and,
thus, the colour, as you can see for two y
Cr3+ complex ions below.
A change in even a single ligand can influence the colour. The [Cr(NH3)6]3+ ion is yellow‐
21
3 6
orange (left); the [Cr(NH3)5CI]2+ ion is purple (right).
Compiled by Denison at Global in Cunupia. 739-2656.
http://www.facebook.com/CAPEChemistryLessons](https://image.slidesharecdn.com/transitionelements-originofcolour-130506210548-phpapp02/75/Transition-Elements-Origin-Of-Colour-21-2048.jpg)


![In 2009 it went like this:
“Use the distribution in the d‐orbitals to
account for colour in transition metal ions.f
[2 marks]”
24Compiled by Denison at Global in Cunupia. 739-2656.
http://www.facebook.com/CAPEChemistryLessons](https://image.slidesharecdn.com/transitionelements-originofcolour-130506210548-phpapp02/75/Transition-Elements-Origin-Of-Colour-24-2048.jpg)
![In 2009 it went like this:
“Use the distribution in the d‐orbitals to
account for colour in transition metal ions.f
[2 marks]”
and in 2007 it went like this:and in 2007 it went like this:
“Account for the origin of colour in transitionAccount for the origin of colour in transition
metal complexes. [4 marks]”
25Compiled by Denison at Global in Cunupia. 739-2656.
http://www.facebook.com/CAPEChemistryLessons](https://image.slidesharecdn.com/transitionelements-originofcolour-130506210548-phpapp02/75/Transition-Elements-Origin-Of-Colour-25-2048.jpg)








