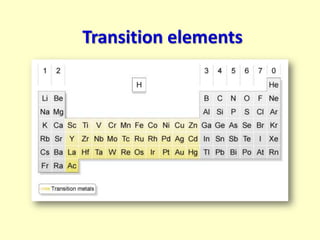
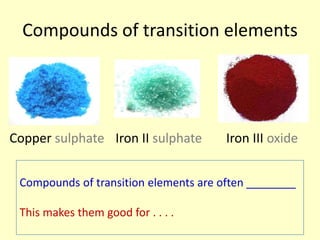
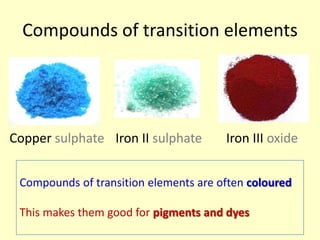
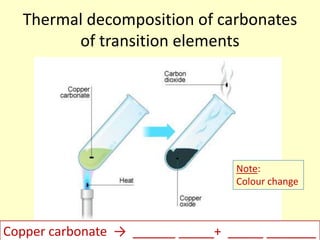
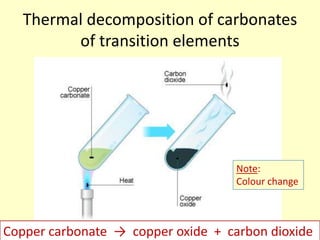
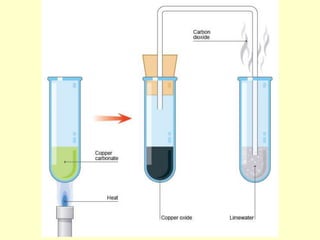
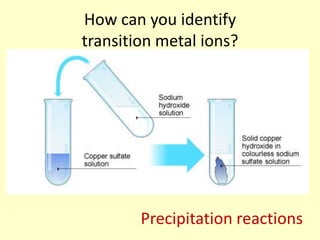
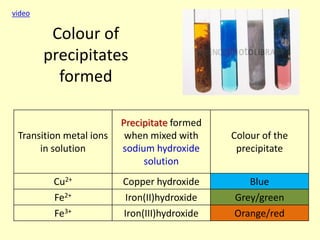
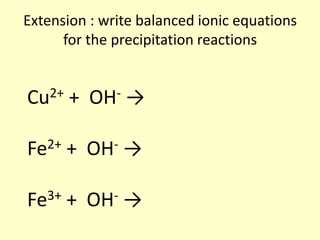
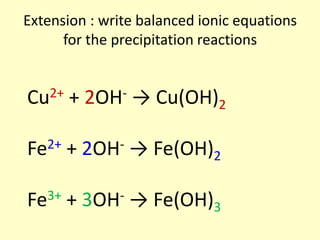
This document discusses transition elements and their compounds. It describes how transition element compounds are often colored, making them useful as pigments and dyes. It also discusses the thermal decomposition of transition metal carbonates into metal oxides and carbon dioxide. Finally, it explains how transition metal ions can be identified through precipitation reactions with sodium hydroxide solution, noting the color of precipitates formed for copper, iron(II), and iron(III) ions.