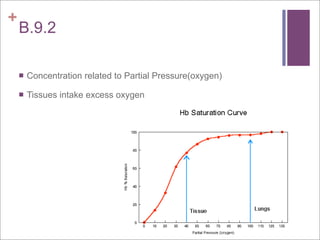
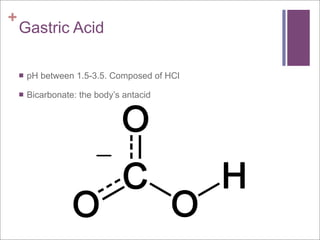
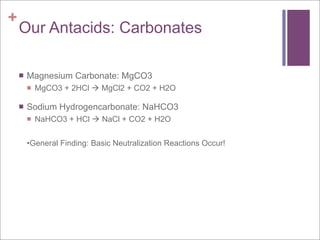
The document compares aerobic and anaerobic respiration, outlining their shared characteristics as well as their differences in efficiency and ATP production. It also discusses the roles of copper and iron ions in electron transport and oxygen transport during respiration. Additionally, it explains how excess acidity in the stomach can be reduced through the use of bases like aluminum hydroxide, magnesium hydroxide, magnesium carbonate, and sodium bicarbonate in antacids. These bases neutralize stomach acid through neutralization reactions.