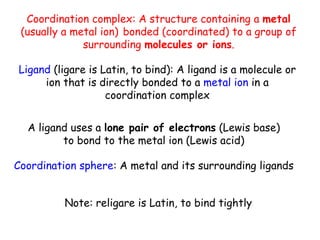
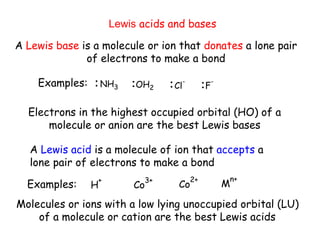
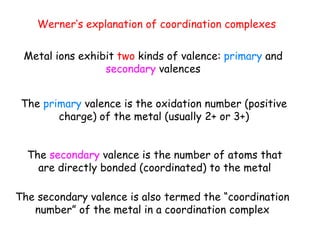
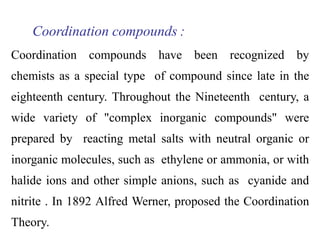
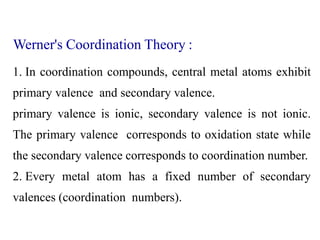
Coordination complexes consist of a central metal ion bonded to surrounding ligand molecules or ions. Alfred Werner established the modern theory of coordination complexes in the late 19th century. According to Werner's theory, the metal ion exhibits both a primary valence based on its oxidation state and a secondary valence equal to its coordination number. Coordination complexes can be named systematically based on the ligands bonded to the metal ion and the metal's identity and oxidation state.


![Conventions in writing the structure of coordination
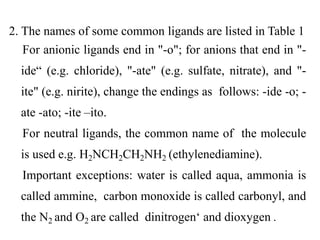
compounds:
Brackets [] are used to indicate all of the
composition of the coordinate complex
The symbol for the central atom metal of the
complex is first within the brackets
Species outside of the [] are not coordinated to the
metal but are require to maintain a charge balance
A coordination compounds is a neutral species
consisting of a coordinate complex and uncoordinated
ions required to maintain the charge balance](https://image.slidesharecdn.com/lec-221224200917-d8d65e33/85/Lec-2-pdf-8-320.jpg)
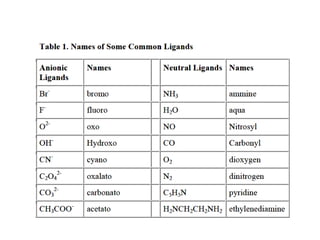
![Ligand substitution reactions
For some complex ions, the coordinated ligands may
be substituted for other ligands
Complexes that undergo very rapid substitution of
one ligand for another are termed labile
Complexes that undergo very slow substitution of
one ligand for another are termed inert
[Ni(H2O)6]2+ + 6 NH3 → [Ni(NH3)6]2+ + 6 H2O (aqueous)](https://image.slidesharecdn.com/lec-221224200917-d8d65e33/85/Lec-2-pdf-9-320.jpg)
![Example of a coordination complex: [Co(NH3)6]Cl3
[Co(NH3)6]3+
What is the atomic
composition of the
complex?
What is the net charge
of the complex?
[Co(NH3)6]
How do we know the charge
is 3+ on the metal?
3+ is required to balance
the three Cl- ions
The primary valence of [Co(NH3)6]Cl3 is 3 (charge on Co)
The secondary valence of [Co(NH3)6]Cl3 is 6 (ligands)](https://image.slidesharecdn.com/lec-221224200917-d8d65e33/85/Lec-2-pdf-11-320.jpg)
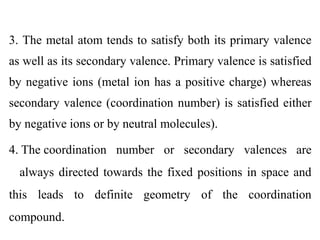
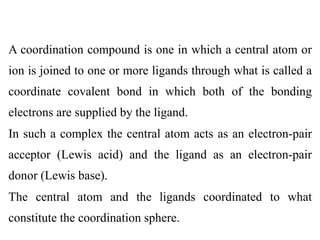
![Thus the salt [Co(NH3)5Cl]Cl2 is composed of the
complex ion [Co(NH3)5Cl]2+ and two Cl– ions;
components within the square brackets are inside the
coordination sphere, whereas the two chloride ions are
situated outside the coordination sphere. These latter two
ions could be replaced by other ions such as NO3
-.](https://image.slidesharecdn.com/lec-221224200917-d8d65e33/85/Lec-2-pdf-16-320.jpg)
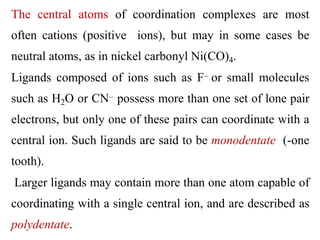
![Thus ethylenediamine is a bidentate ligand.
Polydentate ligands whose geometry enables them to
occupy more than one coordinating position of a central
ion act as chelating agents and tend to form extremely
stable complexes known as chelates.
Coordination sphere: The central atom/ion and the ligands
attached to it are enclosed in square bracket and is
collectively termed as the coordination sphere.
For example: in the complex K4[Fe(CN)6], [Fe(CN)6]4- is
the coordination sphere.](https://image.slidesharecdn.com/lec-221224200917-d8d65e33/85/Lec-2-pdf-18-320.jpg)
![Counter ions: The ions present outside the coordination
sphere are called counter ions.
For example: in the complex K4[Fe(CN)6], K+4 is the
counter ion.
A complex is a structure composed of a central metal atom
or ion, generally a cation, surrounded by a number of
negatively charged ions or neutral molecules possessing
lone pairs. A complex may also be called a coordination
compound or metal complex.](https://image.slidesharecdn.com/lec-221224200917-d8d65e33/85/Lec-2-pdf-19-320.jpg)
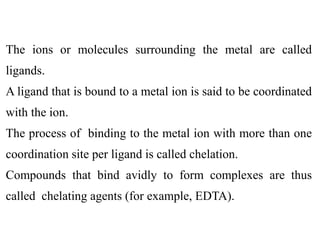

![Donor atom: An atom of the ligand attached directly to the
metal is called the donor atom.
For example: in the complex K4[Fe(CN)6 ] carbon is a
donor atom.
Coordination number: The coordination number (CN) of a
metal ion in a complex can be defined as the number of
ligand donor atoms to which the metal is directly bonded.
For example: in the complex K4[Fe(CN)6], the coordination
number of Fe is 6.](https://image.slidesharecdn.com/lec-221224200917-d8d65e33/85/Lec-2-pdf-22-320.jpg)



![[Co(NH3)6]3+
Hexammine cobalt(III)
K3[Fe(CN)6]
potassium hexacyano ferrate(III)
[Co(NH3)4Cl2]Cl
Tetrammine dichlorocobalt(I) chloride
[FeNO(NH3)5]Cl3
Pentammine nitrosyl iron(III) chloride
[Cr(CO)6]
hexacarbonylchromium(0)
K[V(CO)6]
potassium hexacarbonyl vanadate(I)](https://image.slidesharecdn.com/lec-221224200917-d8d65e33/85/Lec-2-pdf-30-320.jpg)
![1. Do ligands act like Lewis acids or Lewis bases? Why?
2. Do ligands form ionic bonds with the central metal
atom?
3. What are chelating agents?
4. What is a monodentate ligand? example
5. Describe polydentate ligands and provide an example.
6. What are hexadentate ligands?
7. Name this complex [Cu(NH3)4]SO4.
8. Name this complex [Co(en)3](NO3)2.](https://image.slidesharecdn.com/lec-221224200917-d8d65e33/85/Lec-2-pdf-31-320.jpg)
![Q /For the complex K3[Cr(C2O4)3]·3H2O
1)What is the central metal ion?
2)What is its oxidation state?
3)What is its electronic configuration?
4)What is its coordination number?
5)What is the IUPAC name of the complex?
Q/name the following complexes :
[Mn(NO)3CO] , NH4[Cr(NH3)2(NCS)4] ,
[Cu(en)2(H2O)2]SO4](https://image.slidesharecdn.com/lec-221224200917-d8d65e33/85/Lec-2-pdf-32-320.jpg)