This document provides an introduction to coordination compounds. It defines coordination compounds as complex compounds formed when a central metal ion bonds with surrounding neutral or charged ligand species via dative bonds. The central concepts discussed include:
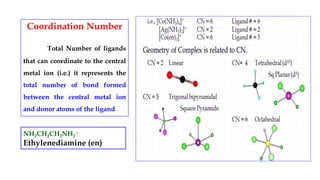
- Coordination number which represents the total number of bonds between the metal ion and ligands.
- Coordination sphere which comprises the metal ion and surrounding ligands.
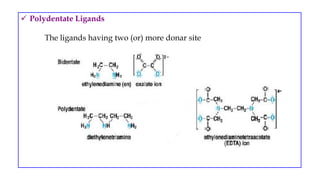
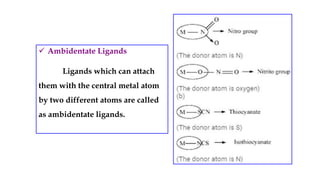
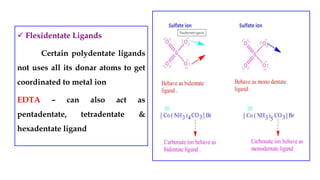
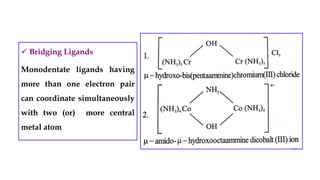
- Ligands which contain lone pair electrons and can be monodentate, polydentate, etc.
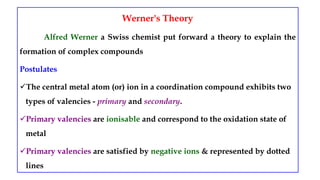
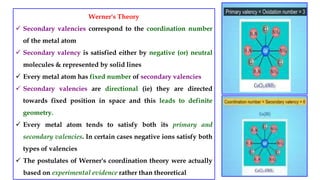
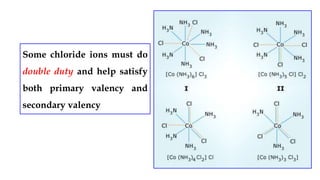
- Werner's theory which proposed that metal ions exhibit both primary and secondary valences satisfied by anions and ligands respectively.
- Modern electronic theories like Sidgwick's effective atomic number rule and valence bond
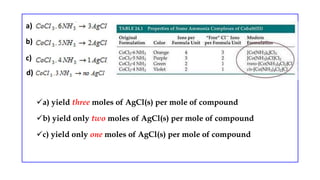
![Double Salts Co-ordination compound
These exist only in solid state and dissociate into
constituent species in their solution
Eg: Mohr’s salt: FeSO4.(NH4)2SO4.6H2O
They retain their identity in solid as well in
solution state
They lose their identity in dissolved state
Eg: An aqueous solution of potash alum will give
the tests for K+, Al3+, and SO4
-2
K2SO4.Al2(SO4)3.24H2O →2K++ 2Al+3 + 4SO4
-2+ 24H2O
They do not lose their identity in dissolved state
Their properties are essentially the same as those of
constituent species
Their properties are different from those of their
constituents
Eg: K4[Fe(CN)6] does not show the test of Fe2+ and
CN- ions](https://image.slidesharecdn.com/coordinationcompoundsuptovbt2-240123094510-bb7d1bbc/85/Coordination-compounds-upto-VBT-2-pptx-3-320.jpg)
![Coordination Compounds
• The central metal ion in the complex forms dative (or) coordinate
covalent bonds with the neutral/anionic/cationic species surrounding it
[Fe(CN)6]4-](https://image.slidesharecdn.com/coordinationcompoundsuptovbt2-240123094510-bb7d1bbc/85/Coordination-compounds-upto-VBT-2-pptx-4-320.jpg)
![Coordination Sphere
[Fe(CN)6]3- - Comprises of metal ion and ligands
Oxidation State
K3[Fe(CN)6] = 3K+ + [Fe(CN)6]3- [Co(NH3)4Cl2]Cl = [Co(NH3)4Cl2]1+ + Cl-
Oxidation state of Fe = 3 Oxidation state of Co = 3
X + 6 (-1) = -3 X +4 (0)+2 (-1) = +1
X = -3+6 X = +1+2
= 3 = 3](https://image.slidesharecdn.com/coordinationcompoundsuptovbt2-240123094510-bb7d1bbc/85/Coordination-compounds-upto-VBT-2-pptx-6-320.jpg)


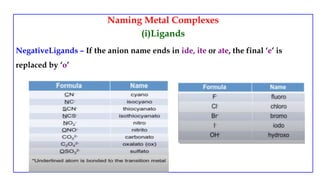


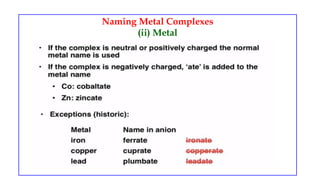

![Naming Metal Complexes
• Counter ions are named as separate words and are not numbered
Anionic Complex
[Cr(en)I4]- - ethylenediamminetetraiodochromate(III) ion
[Zn(OH)4]2- - tetrahydroxozincate(II) ion
K3[Co(CN)6] - potassium hexacyanocobaltate(III)
Cationic Complex
[Ag(NH3)2]+ - diamminesilver(I) ion
[Co(NH3)4Cl2]Cl – tetraamminedichlorocobalt(III) chloride
Neutral Complex
[Ni(CO)4] - tetracarbonylnickel(0)](https://image.slidesharecdn.com/coordinationcompoundsuptovbt2-240123094510-bb7d1bbc/85/Coordination-compounds-upto-VBT-2-pptx-18-320.jpg)
![1. [Cr(NH3)3(H2O)3]Cl3
triamminetriaquachromium(III) chloride
2. [Pt(NH3)5Cl]Br3
pentaamminechloroplatinum(IV) bromide
3. [Pt(H2NCH2CH2NH2)2Cl2]Cl2
dichlorobis(ethylenediamine)platinum(IV) chloride
4. Na2[NiCl4]
sodium tetrachloronickelate(II)
4. [Ag(NH3)2][Ag(CN)2] - diamminesilver(I) dicyanoargentate(I)](https://image.slidesharecdn.com/coordinationcompoundsuptovbt2-240123094510-bb7d1bbc/85/Coordination-compounds-upto-VBT-2-pptx-19-320.jpg)


![Example 1
EAN of Fe (II) in [Fe(CN)6]4-
Fe (0) atom Z= 26 electrons
Fe (II) ion (Z-X) = 26-2 = 24
Electrons donated by
6 CN- = Y = 2X6 = 12
EAN = Z – X + Y
= 24 + 12 = 36 (Kr)
Example 2
EAN of Pt (IV) in [Pt(NH3)6]4+
Pt (0) atom Z = 78 electrons
Pt (IV) ion (Z-X) = 78 – 4 = 74
Electrons donated by
6 NH3 = Y = 2 X 6 = 12
EAN = Z – X + Y
= 74 + 12 = 86 (Rn)](https://image.slidesharecdn.com/coordinationcompoundsuptovbt2-240123094510-bb7d1bbc/85/Coordination-compounds-upto-VBT-2-pptx-27-320.jpg)

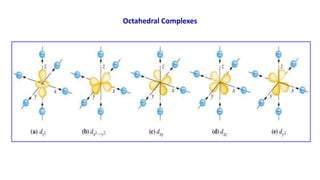

![Example:
[Fe(CN)6]3- - Hexacyanoferrate(III) ion (paramagnetic with one unpaired electron)
Fe = 3d6 4s2 4p0 & Fe3+ = 3d5 4s0 µ = √n(n+2) BM = √1(1+2) = 1.73 BM
Fe3+
Fe3+ in [Fe(CN)6]3-
[Fe(CN)6]3-
Octahedral d2sp3 Geometry
↑↓ ↑↓ ↑
xx xx xx xx
↑ ↑ ↑ ↑ ↑
mix
d2sp3
↑↓ ↑↓ ↑ xx xx
3d 4s 4p
3d
3d
4s
4s
4p
4p
dx
2-y
2 dz
2
dx
2-y
2 dz
2](https://image.slidesharecdn.com/coordinationcompoundsuptovbt2-240123094510-bb7d1bbc/85/Coordination-compounds-upto-VBT-2-pptx-33-320.jpg)
![Octahedral sp3d2 Geometry
Example:
[CoF6]3- - Hexafluorocobaltate (III) ion (paramagnetic with four unpaired electron)
Co = 3d7 4s2 4p0 & Co3+ = 3d6 4s0 µ = √n(n+2) BM
Co3+
Co3+ in [CoF6]3-
[CoF6]3-
↑↓ ↑ ↑ ↑ ↑
xx xx xx xx
↑↓ ↑ ↑ ↑ ↑
mix
sp3d2
↑↓ ↑ ↑ ↑ ↑
4dz
2 4dx
2-y
2
3d 4s 4p
3d 4s 4p
3d
4s 4p
xx xx
4dz
2 4dx
2-y
2
dx
2-y
2 dz
2
dx
2-y
2 dz
2](https://image.slidesharecdn.com/coordinationcompoundsuptovbt2-240123094510-bb7d1bbc/85/Coordination-compounds-upto-VBT-2-pptx-34-320.jpg)
![Tetrahedral sp3 Geometry
Example:
[MnCl4]2- (paramagnetic with five unpaired electron)
Mn = 3d5 4s2 4p0 & Mn2+ = 3d54s0 µ = √n(n+2) BM = √5(5+2) = 5.9 BM
Mn2+
Mn2+ in [MnCl4]2-
[MnCl4]2-
↑ ↑ ↑ ↑ ↑
xx xx xx xx
↑ ↑ ↑ ↑ ↑
mix
sp3
↑ ↑ ↑ ↑ ↑
3d 4s 4p
3d 4s 4p
3d
4s 4p](https://image.slidesharecdn.com/coordinationcompoundsuptovbt2-240123094510-bb7d1bbc/85/Coordination-compounds-upto-VBT-2-pptx-35-320.jpg)
![Square planar dsp2 Geometry
Example:
[Ni(CN)4]2- (Diamagnetic)
Ni = 3d8 4s2 4p0 & Ni2+ = 3d8 4s0
Ni2+
Ni2+ in [Ni(CN)4]2-
[Ni(CN)4]2-
↑↓ ↑↓ ↑↓ ↑↓
xx xx xx
↑↓ ↑↓ ↑↓ ↑ ↑
mix
dsp2
↑↓ ↑↓ ↑↓ ↑↓ xx
3d 4s py
3d 4s 4p
3d
4s 4p
3 dx
2-y
2 px
4p](https://image.slidesharecdn.com/coordinationcompoundsuptovbt2-240123094510-bb7d1bbc/85/Coordination-compounds-upto-VBT-2-pptx-36-320.jpg)
![Example:
[Cu(NH3)4]2+
Ni = 3d9 4s2 4p0 & Cu2+ = 3d9 4s0
a) Cu2+
b) Cu2+ in [Cu(NH3)4]2+
c) Cu2+ in [Cu(NH3)4]2+
↑↓ ↑↓ ↑↓ ↑↓ ↑
3d 4s 4p
↑↓ ↑↓ ↑↓ ↑↓ ↑
3d 4s 4p pz
↑↓ ↑↓ ↑↓ ↑↓
3d 4s 4p
↑
5s
↑↓ ↑↓ ↑↓ ↑↓ ↑ xx xx xx
3d 4s 4p
xx
5d
dx
2-y
2
sp2d](https://image.slidesharecdn.com/coordinationcompoundsuptovbt2-240123094510-bb7d1bbc/85/Coordination-compounds-upto-VBT-2-pptx-37-320.jpg)
