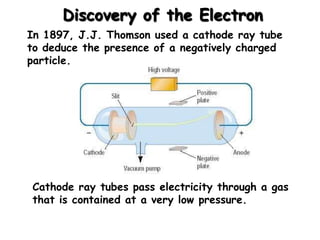
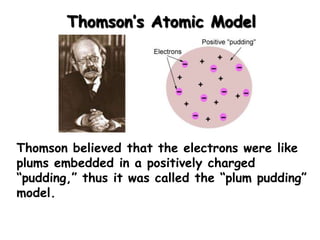
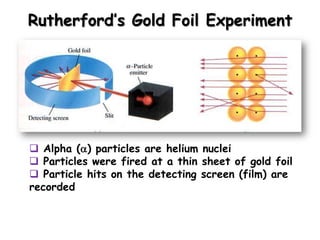
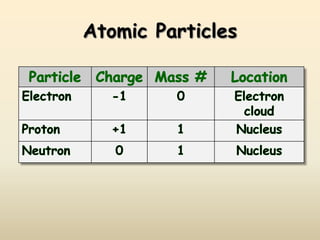
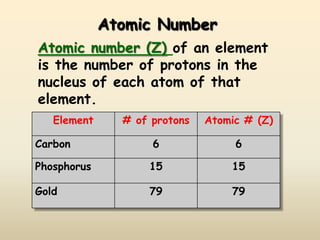
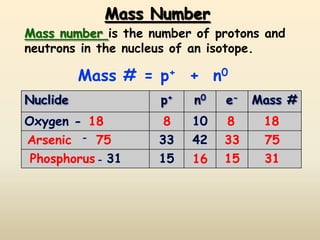
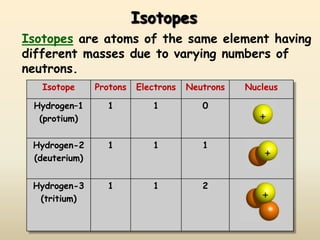
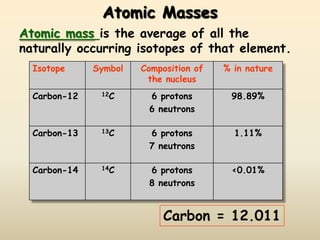
The document discusses the history and development of atomic structure and models. It describes J.J. Thomson's discovery of the electron using cathode ray tubes in 1897. Rutherford's gold foil experiment in the early 1900s showed that the atom has a small, dense, positively charged nucleus. The key parts of an atom - protons, neutrons, electrons - and their roles are defined. Atomic number identifies the number of protons in an element, and mass number identifies the total protons and neutrons in an isotope.