The document discusses key concepts about solutions, including:
1) The factors that determine whether a substance will dissolve and how much will dissolve include the nature of the solute and solvent, stirring, surface area, and temperature.
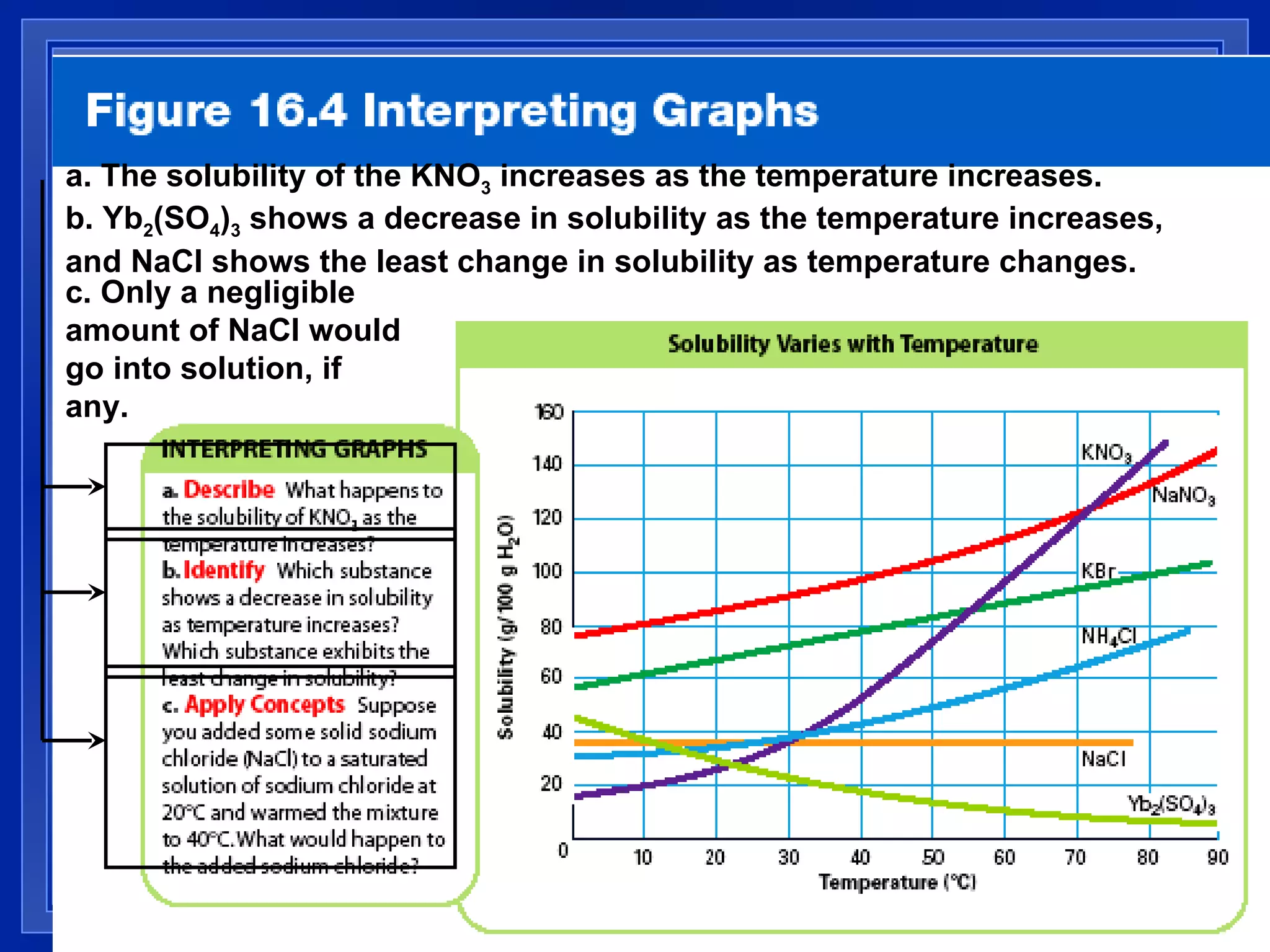
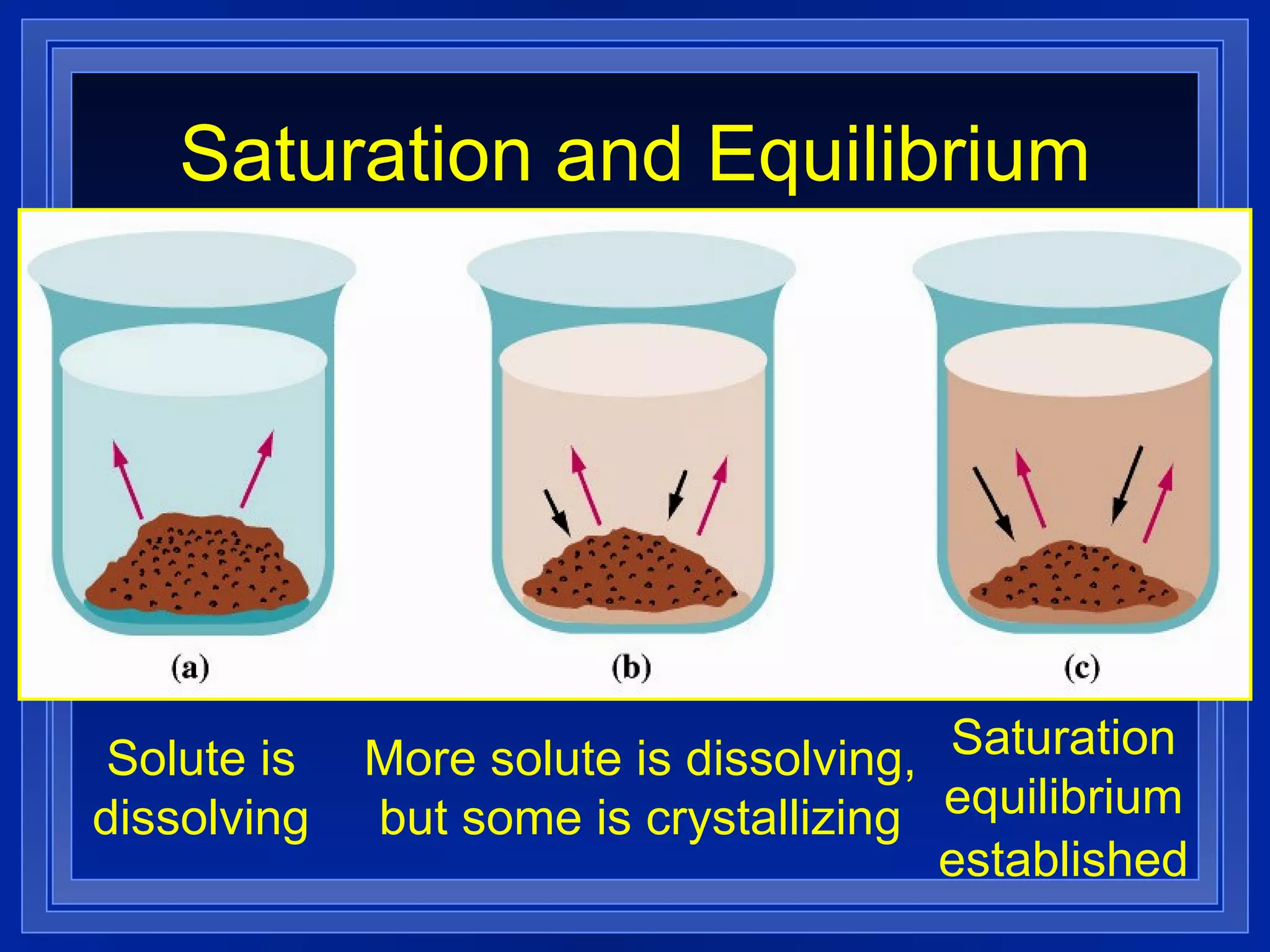
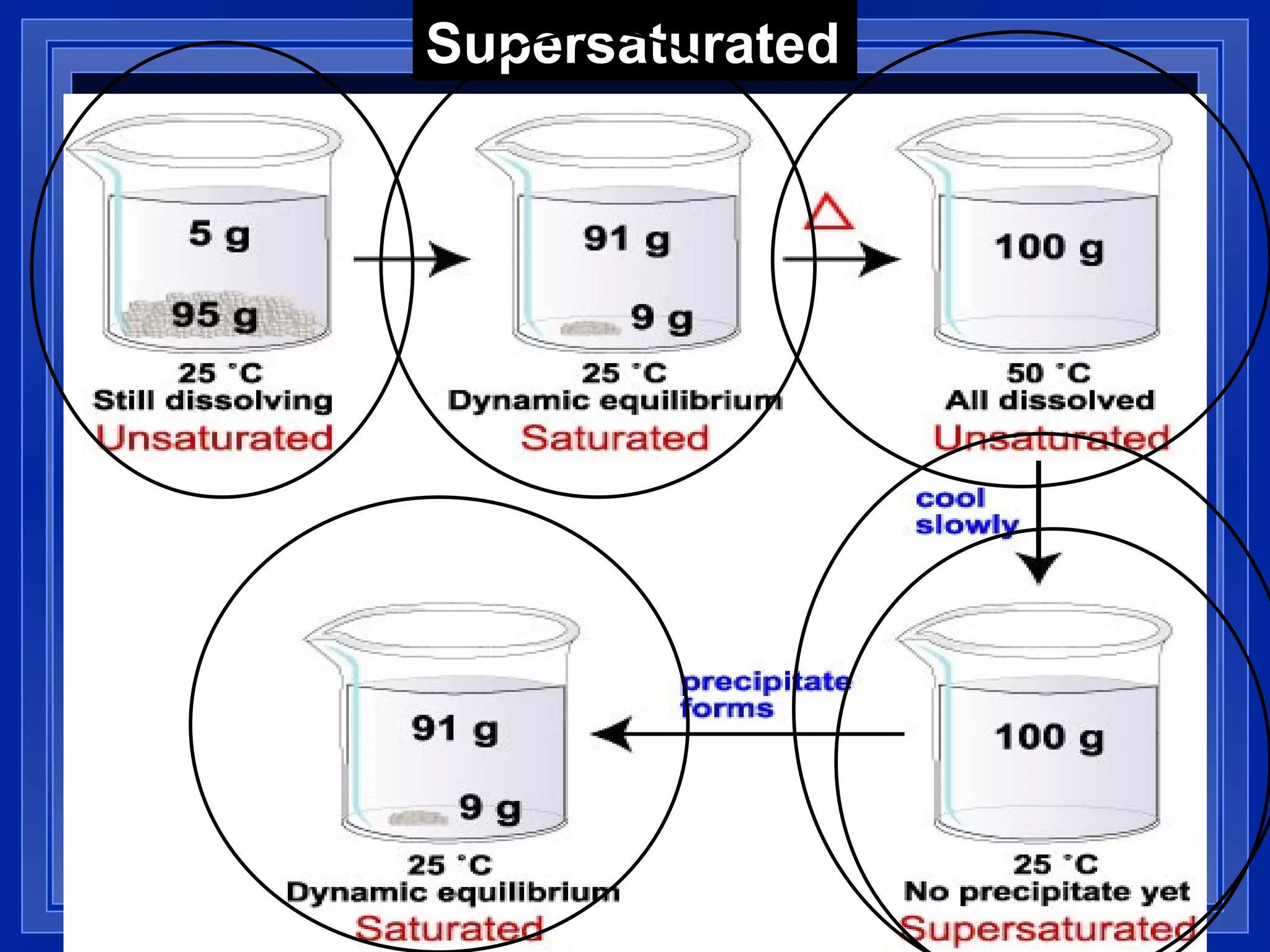
2) Solubility is defined as the maximum amount of solute that will dissolve at a specific temperature, and can be expressed in units like grams of solute per 100 grams of solvent.
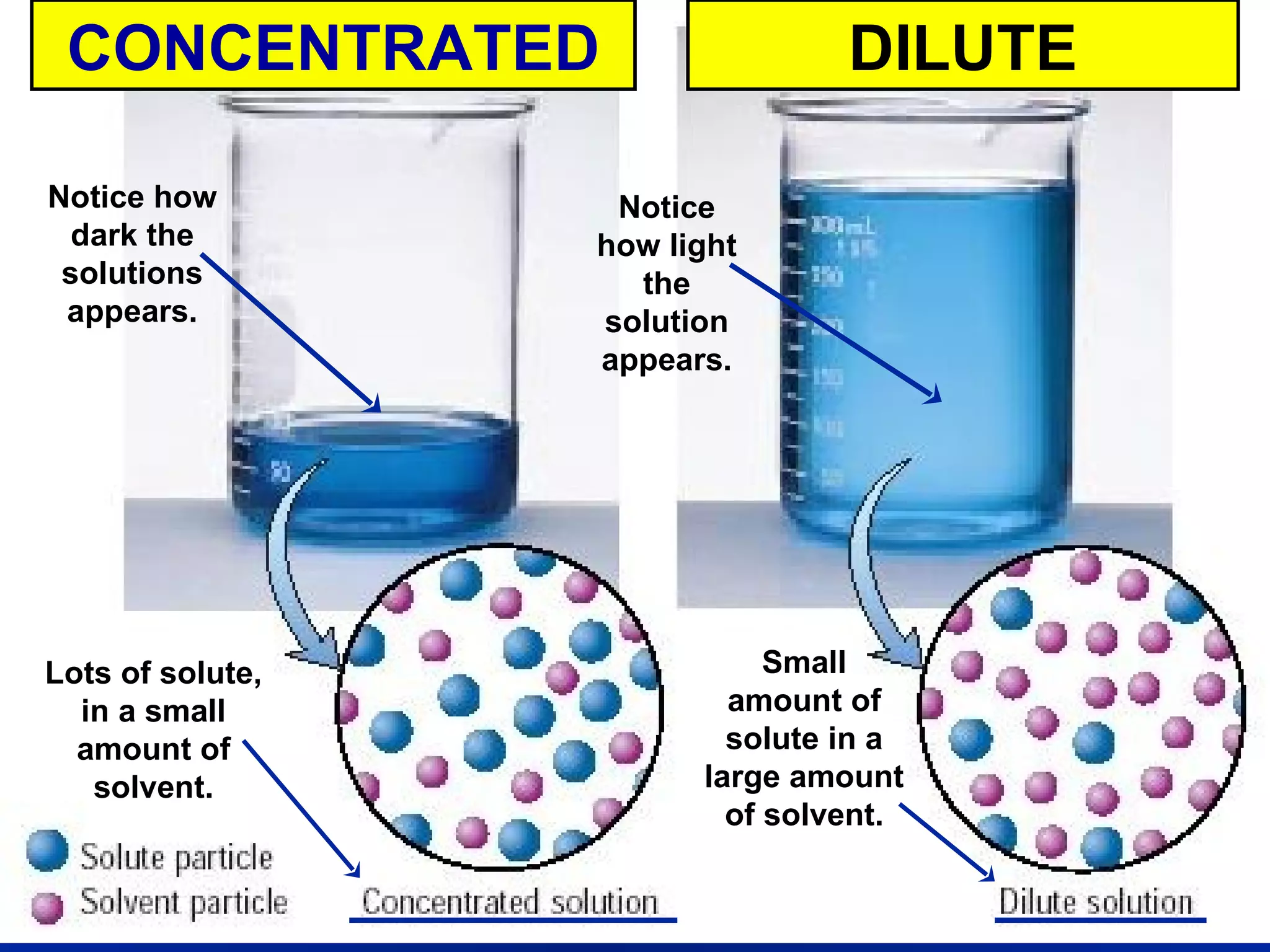
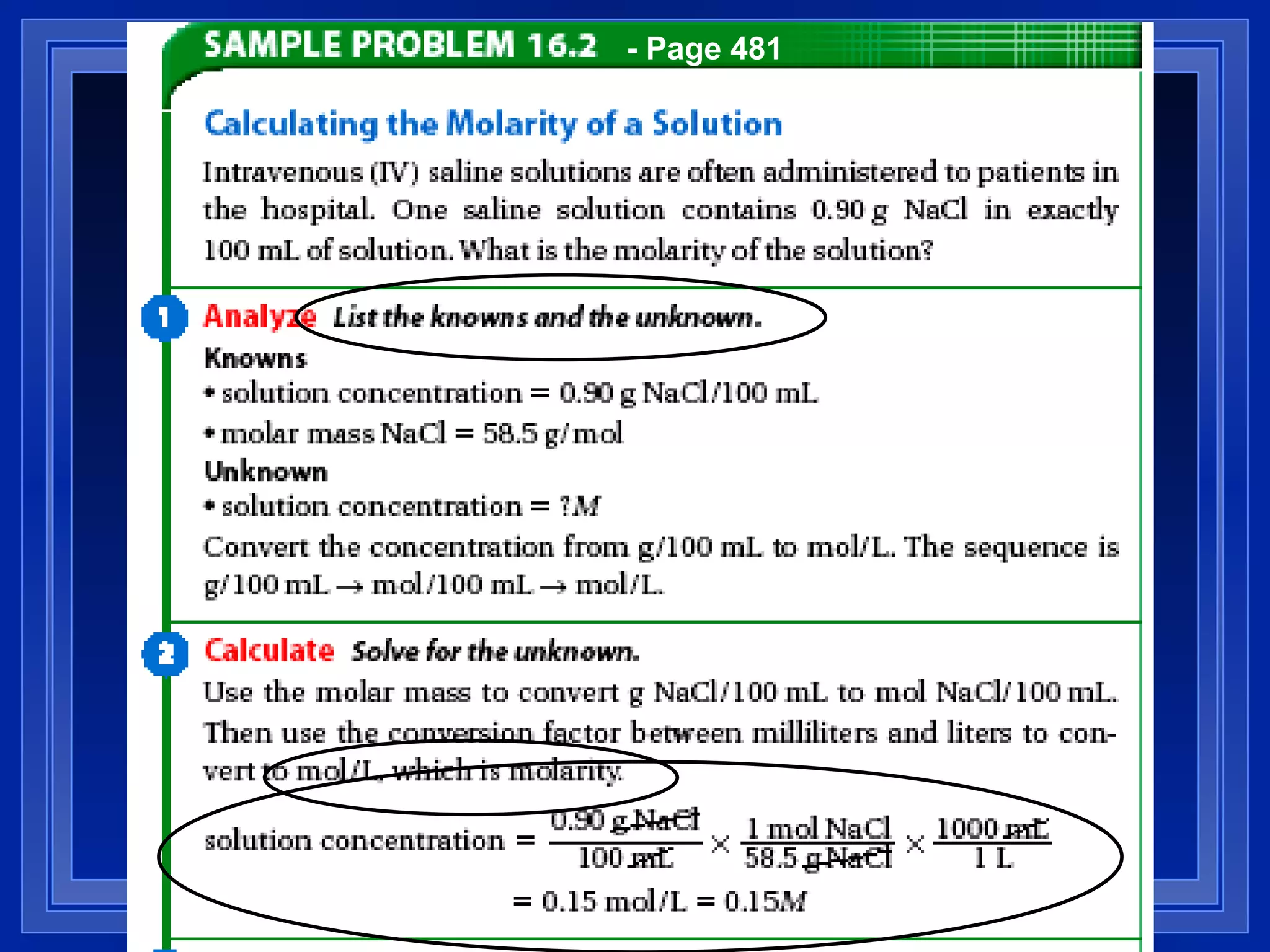
3) Concentration of a solution can be quantified using molarity, which is defined as moles of solute per liter of solution. More concentrated solutions have a larger amount of solute per amount of solvent.