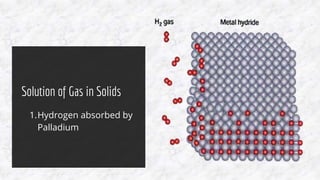
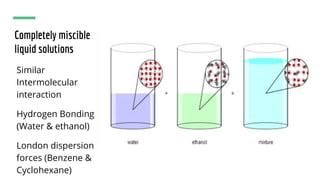
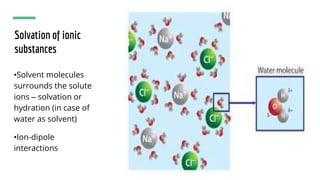
The document discusses the characteristics of solutions and colloids, defining key terms such as solute, solvent, and types of solutions based on their phases. It outlines factors that affect solubility and dissolution rates, including particle size, temperature, concentration, and stirring, as well as explaining the solvation processes for ionic and molecular substances. Additionally, it highlights daily life applications of heat of solution, such as hot and cold packs.