Embed presentation
Downloaded 18 times
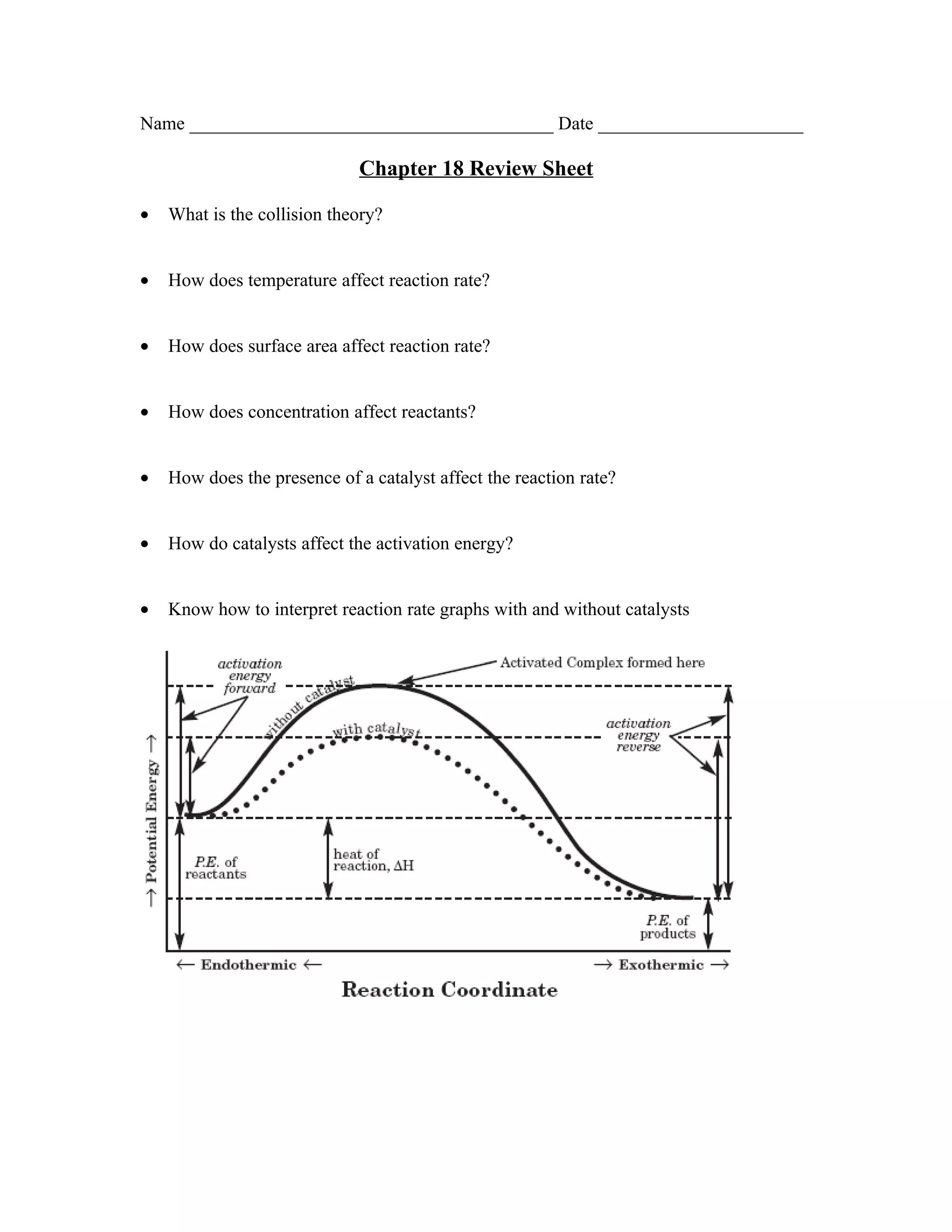

The document is a review sheet for Chapter 18 that covers several key concepts: - The collision theory and how temperature, surface area, concentration, and catalysts affect reaction rates - Equilibrium, LeChatlier's principle, and how concentration, temperature, and pressure shifts affect equilibrium - The definitions of endothermic and exothermic reactions and how to calculate heat of reaction - How to calculate equilibrium constants and how they determine favored products or reactants - The definitions of entropy and enthalpy and how they relate to spontaneous reactions

