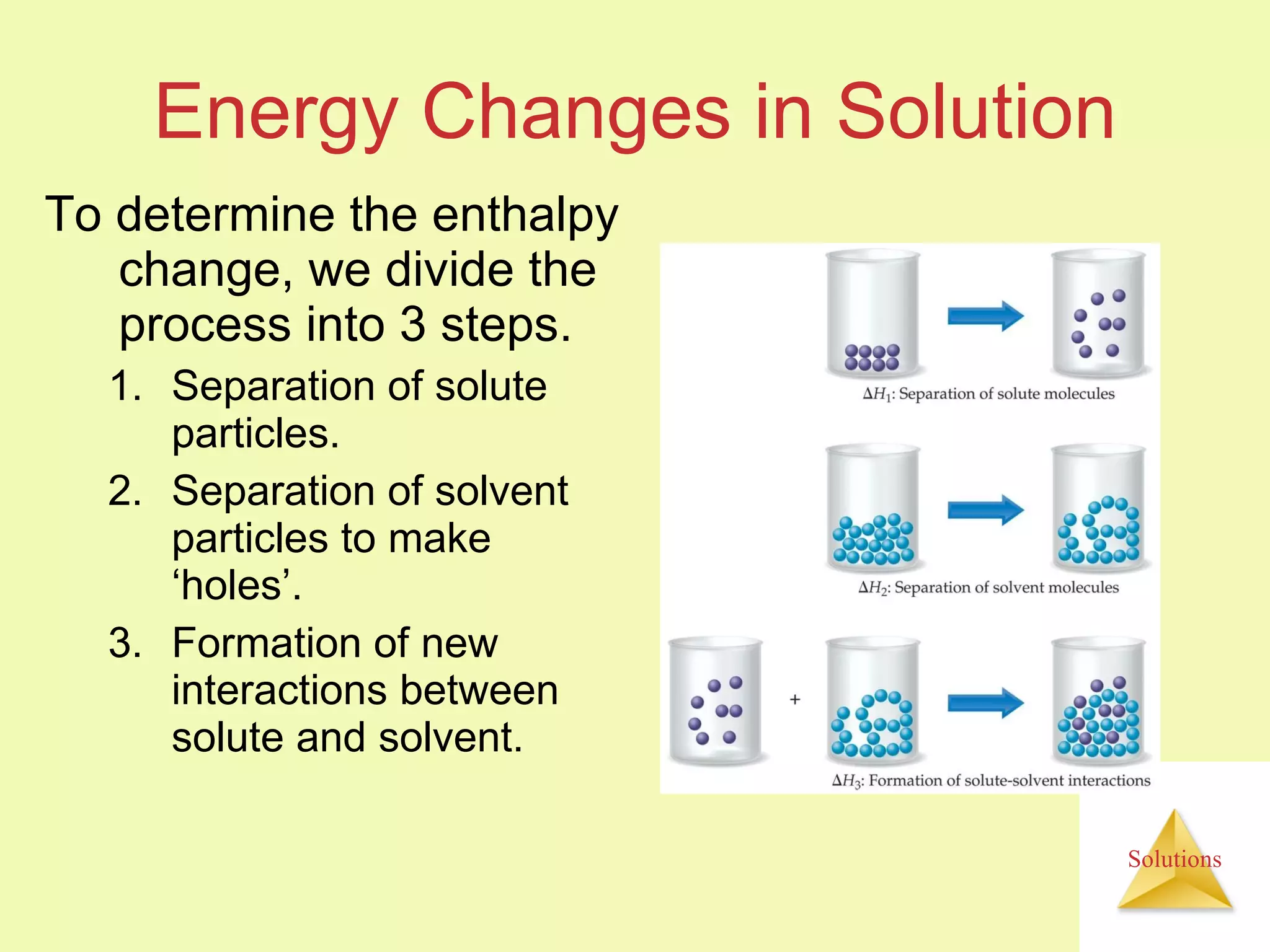
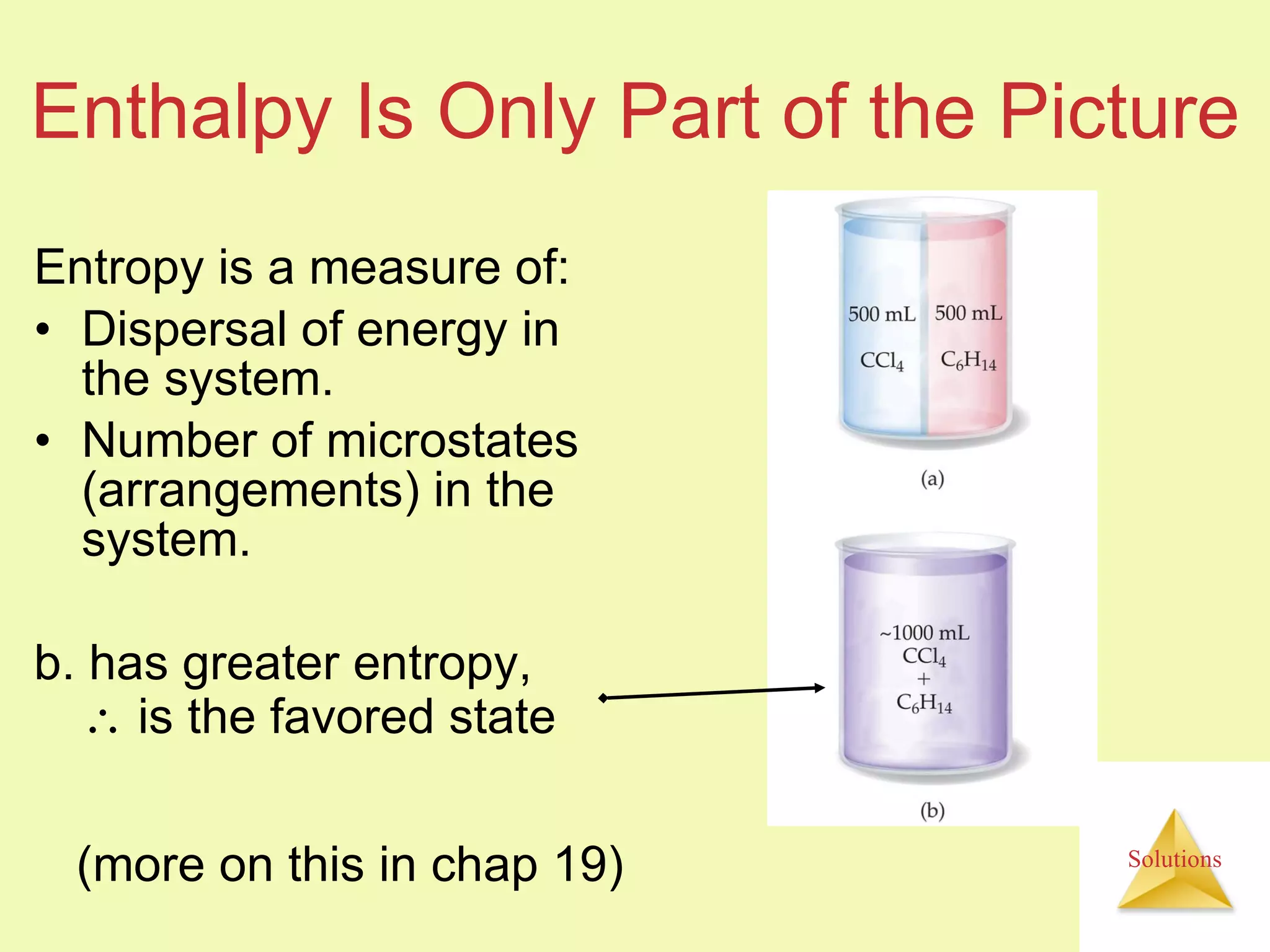
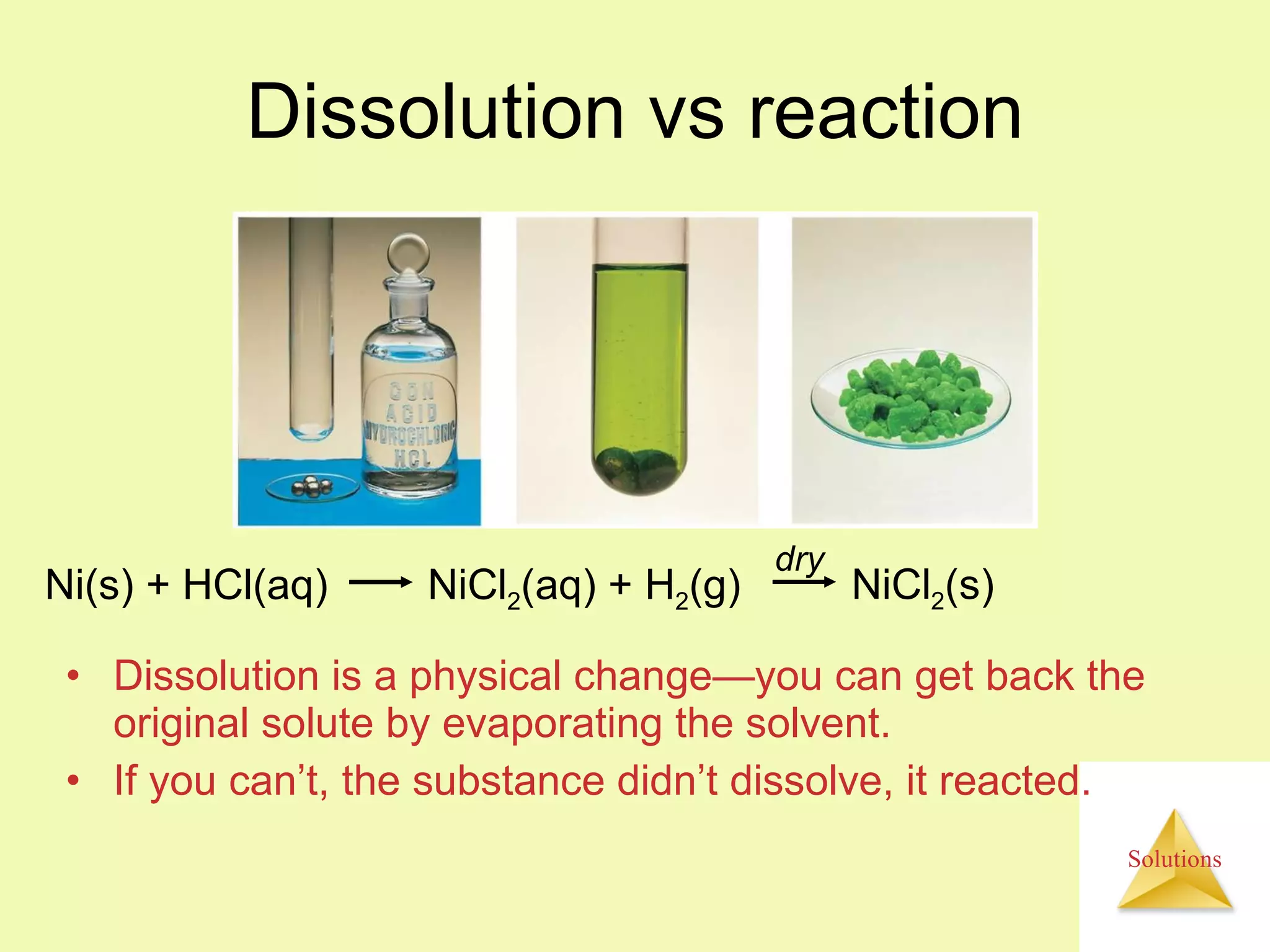
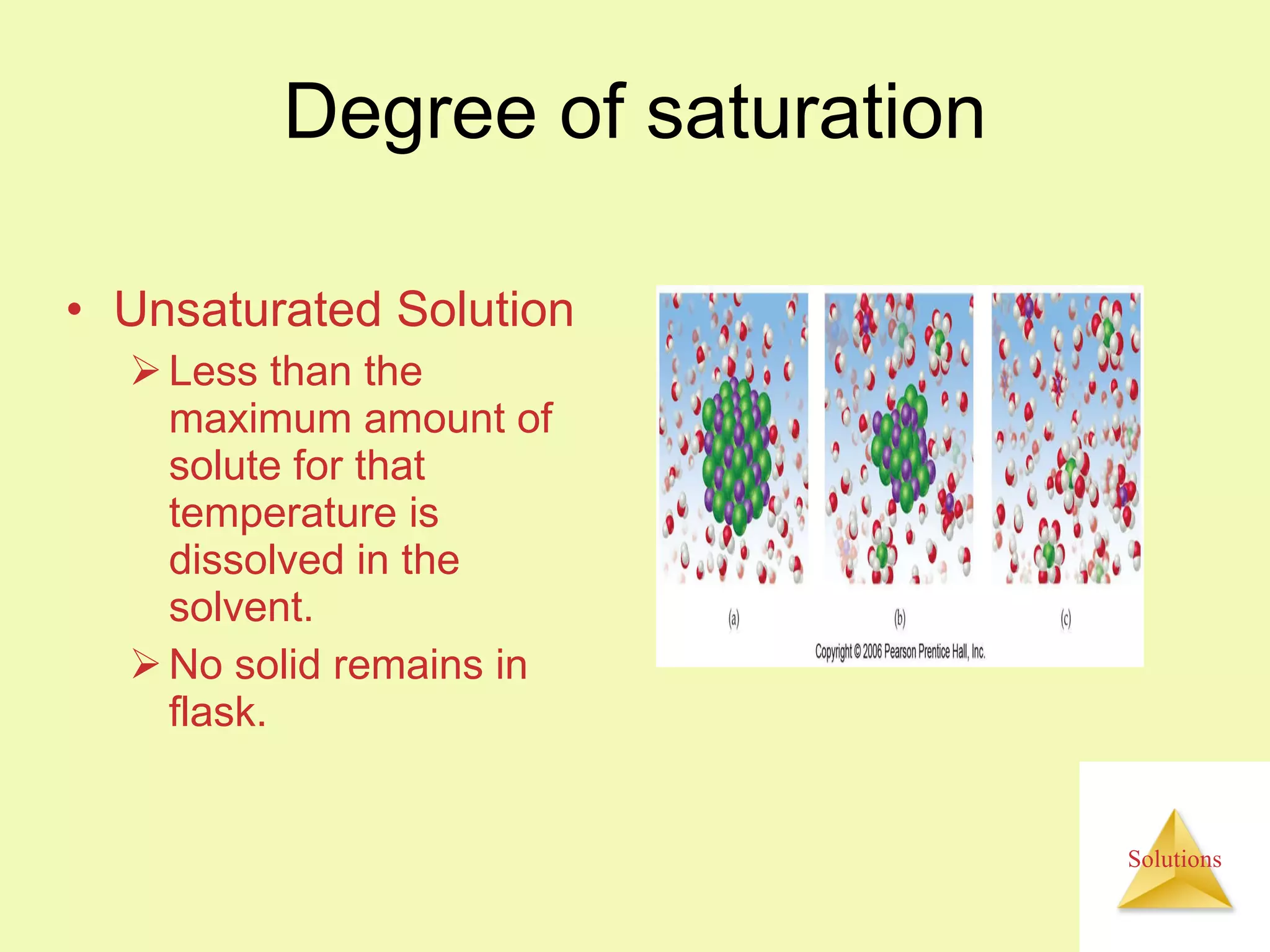
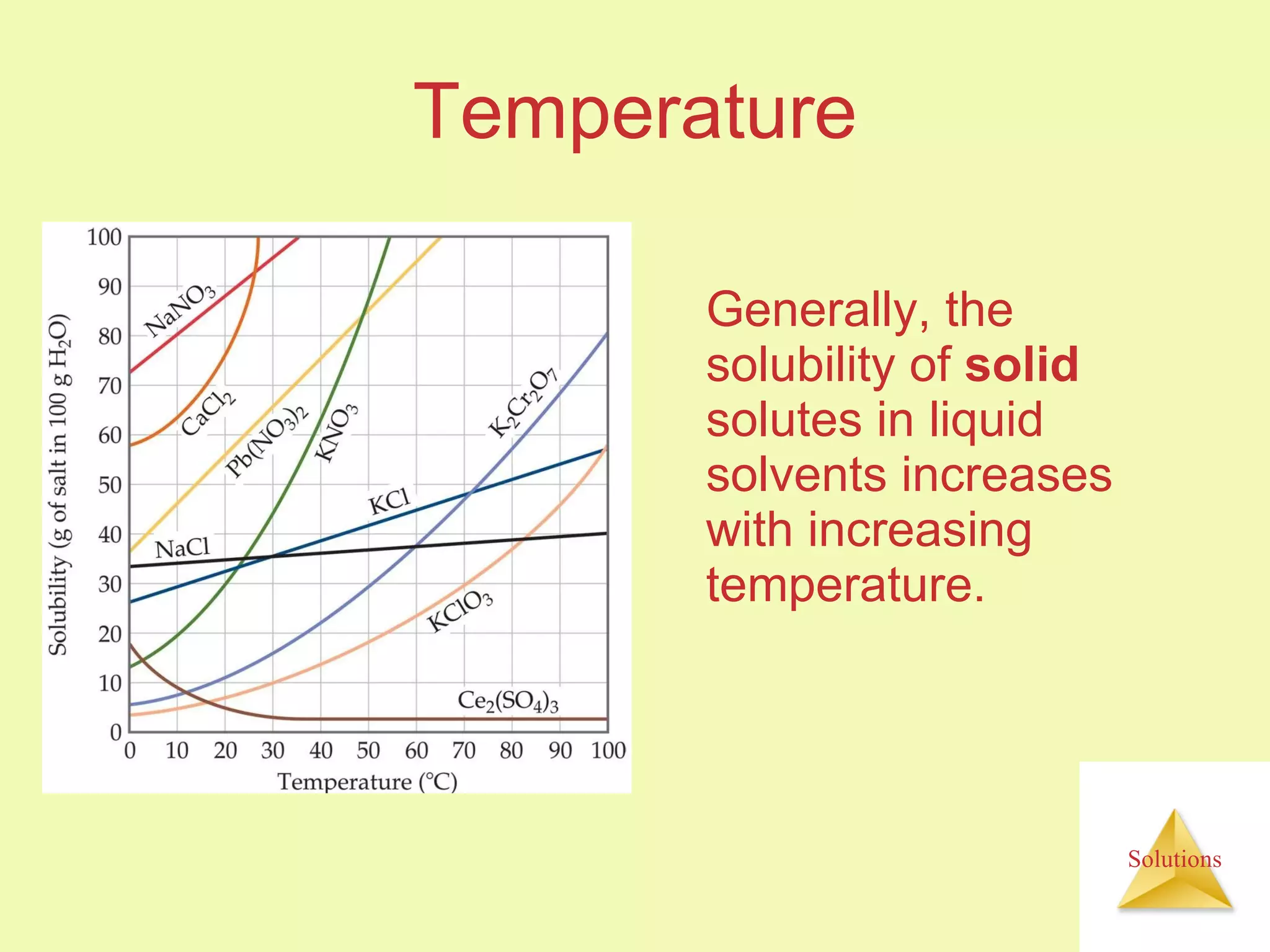
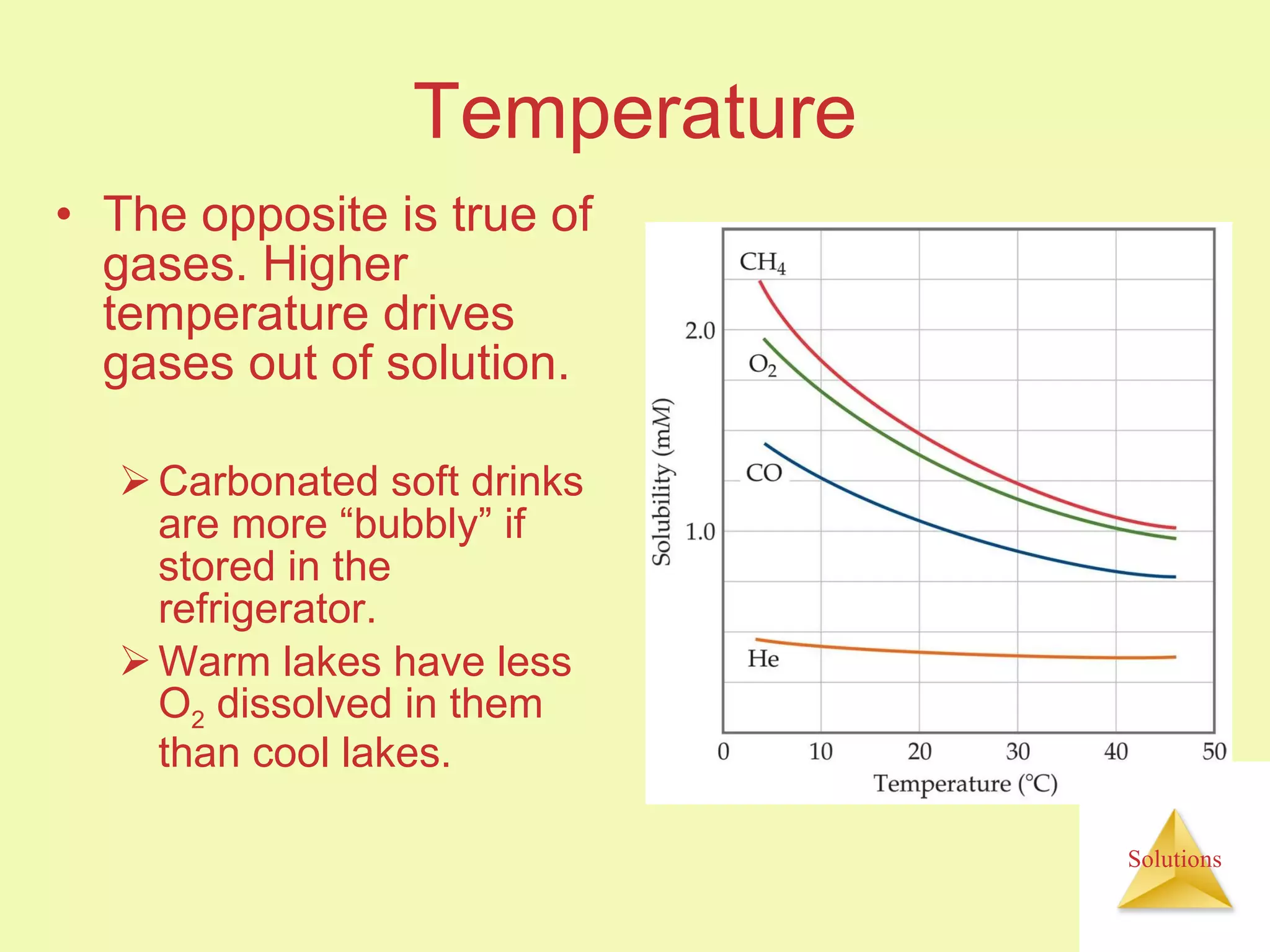
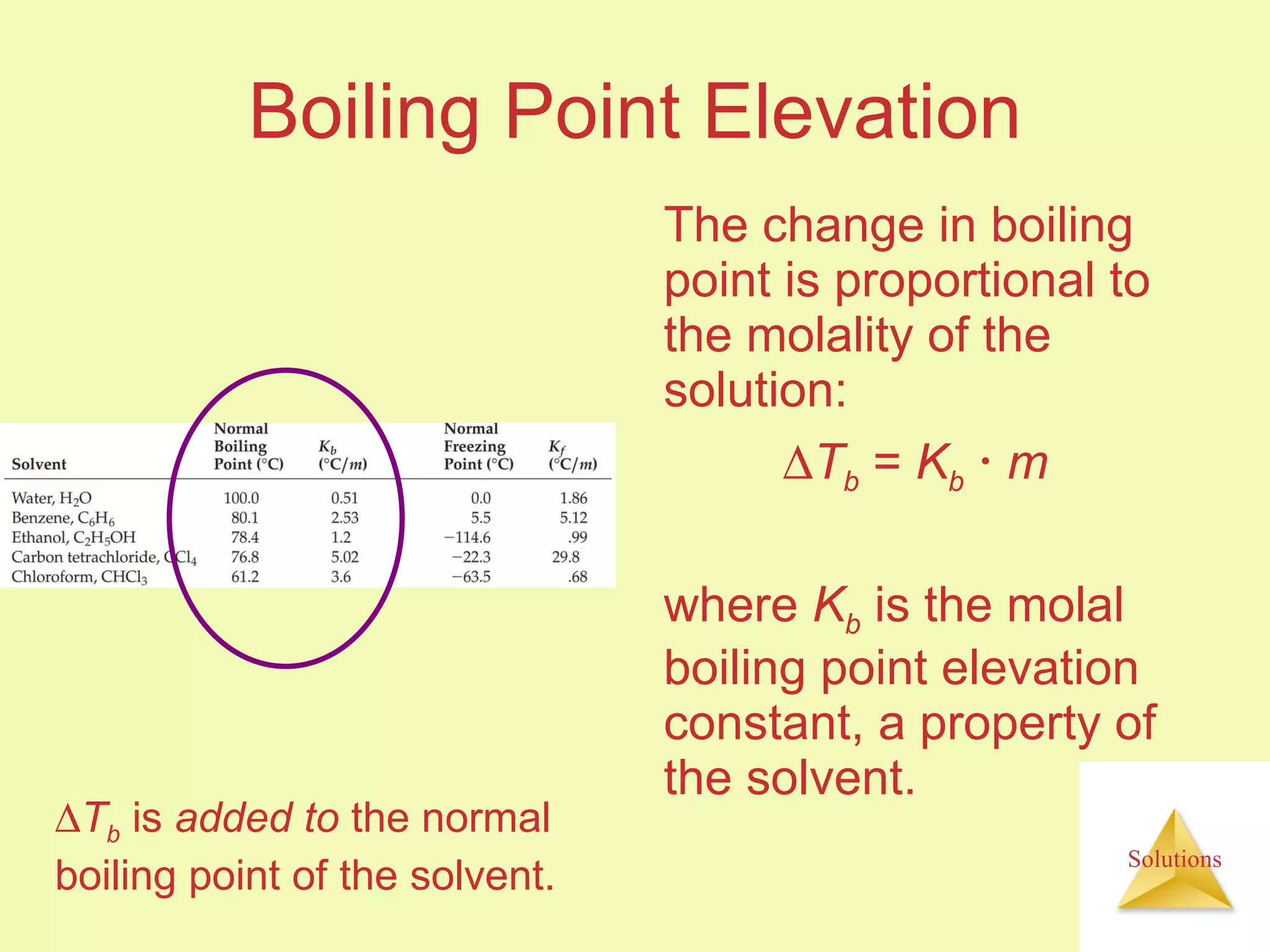
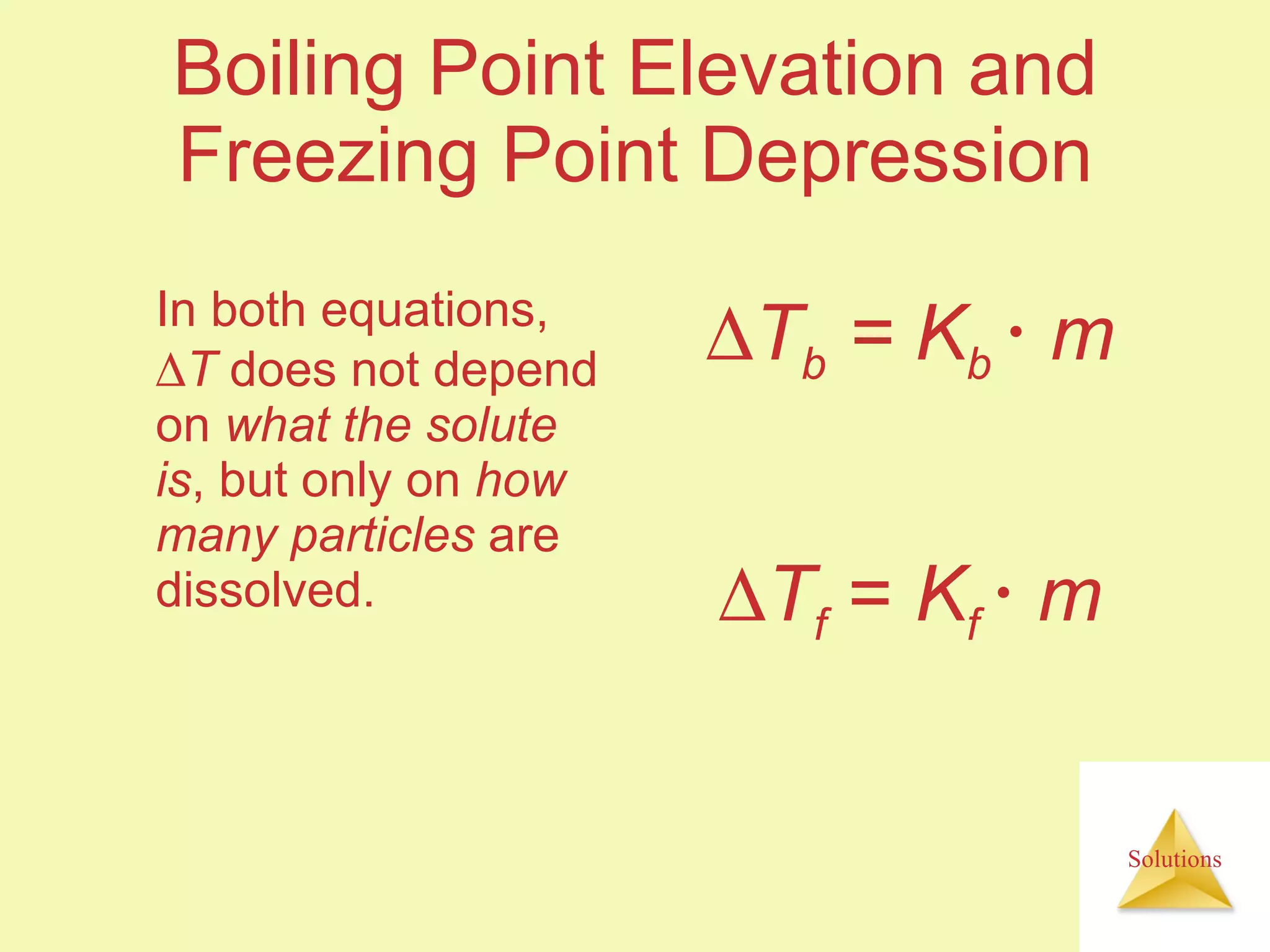
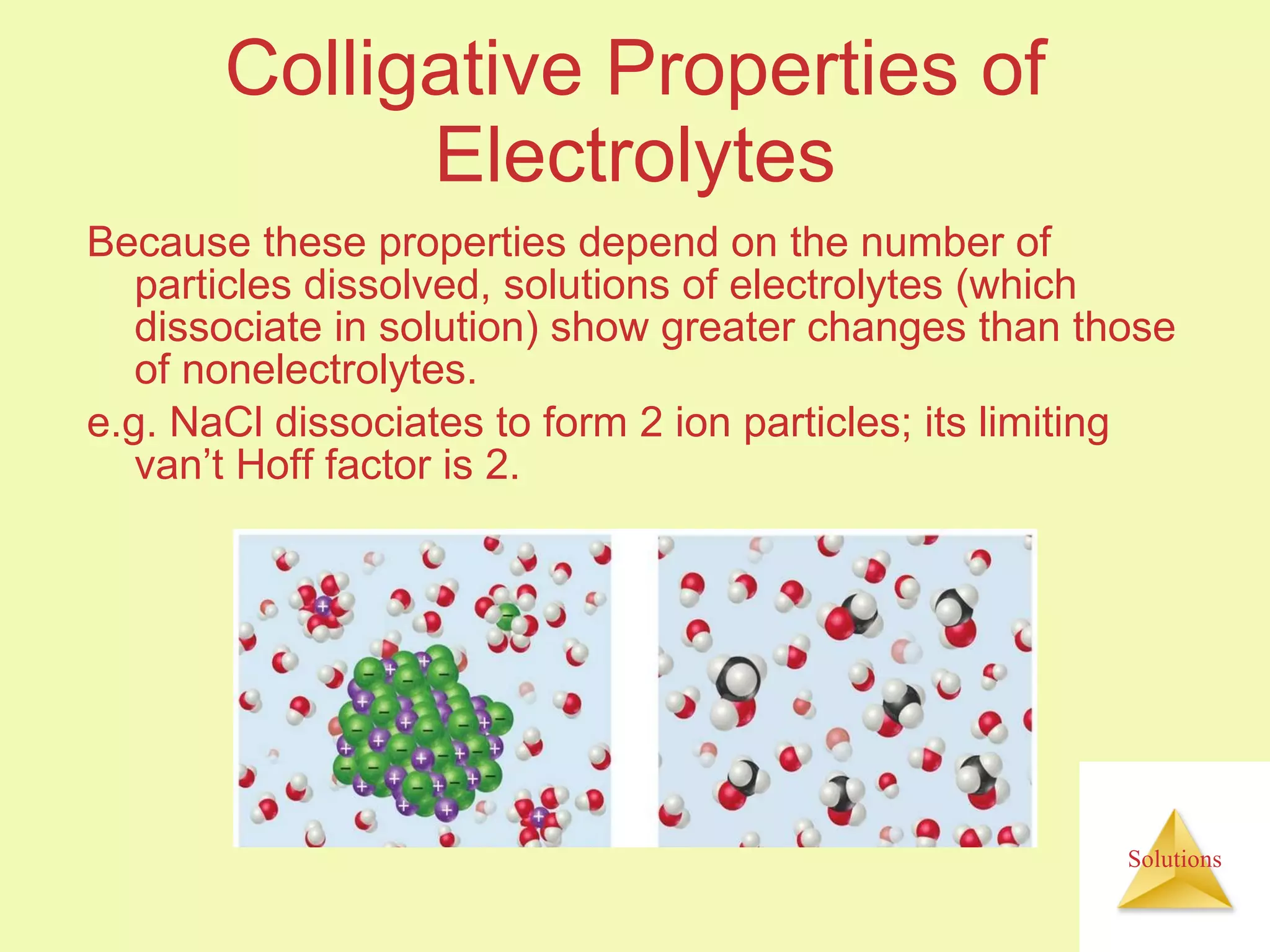
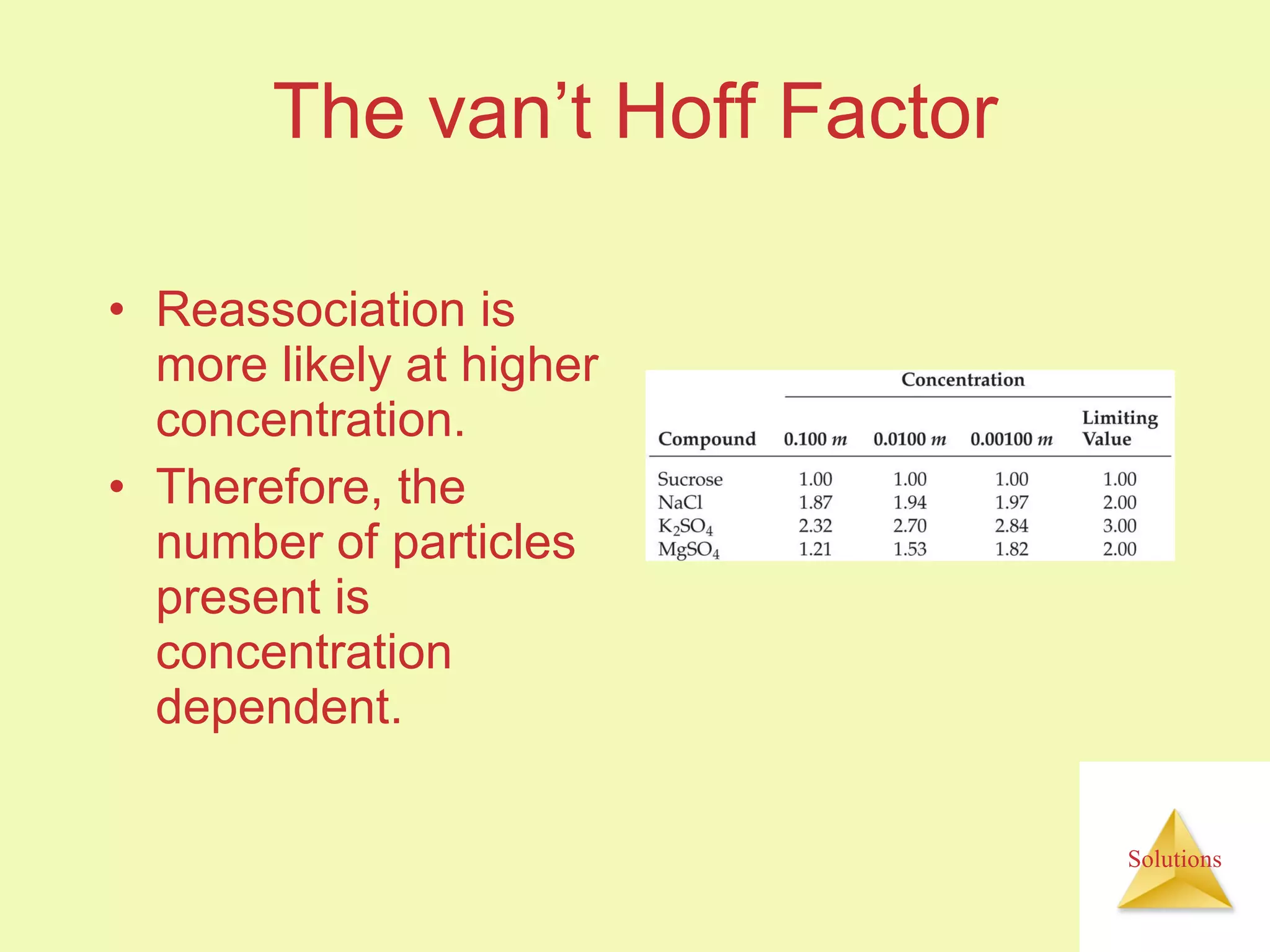
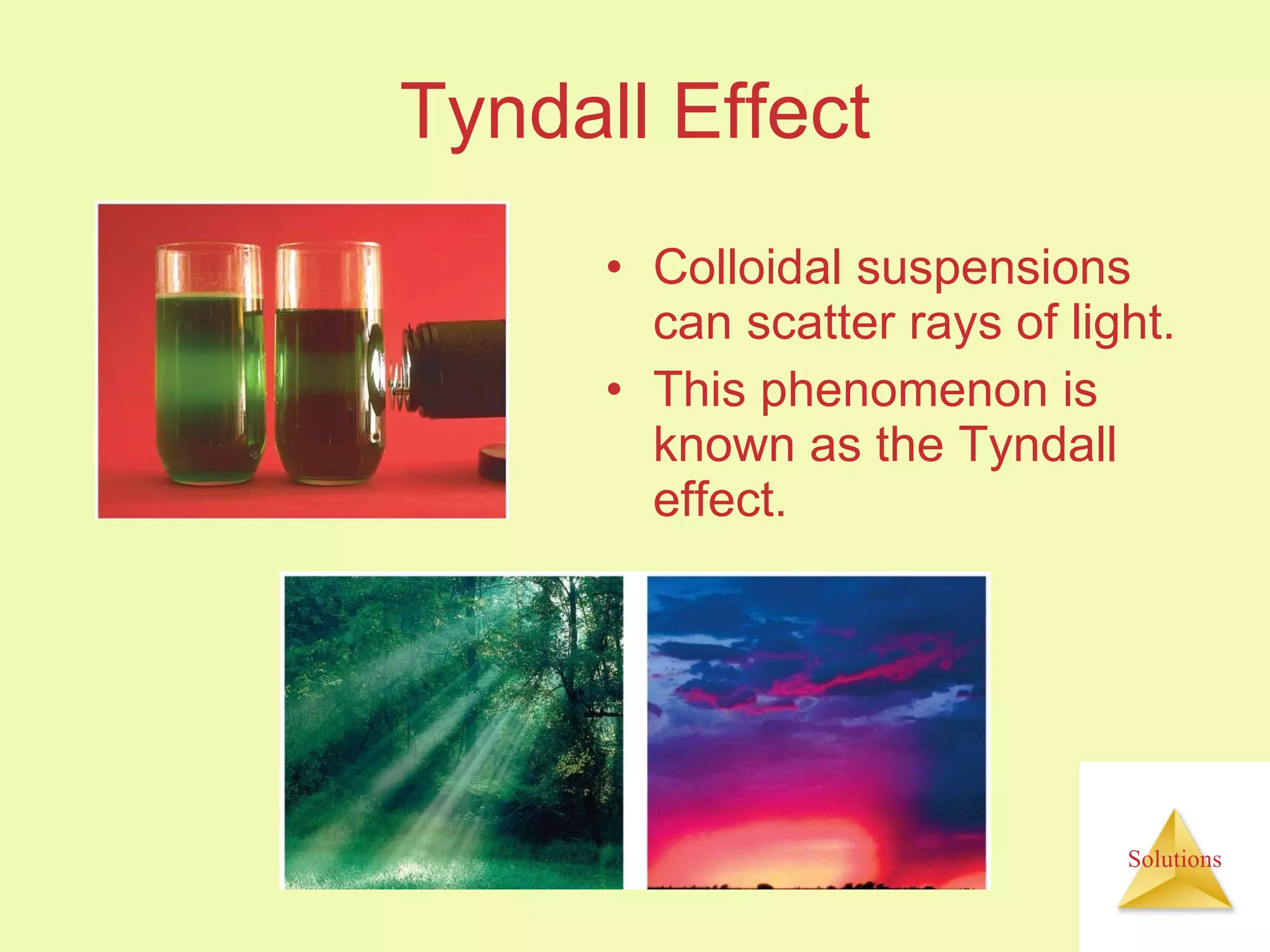
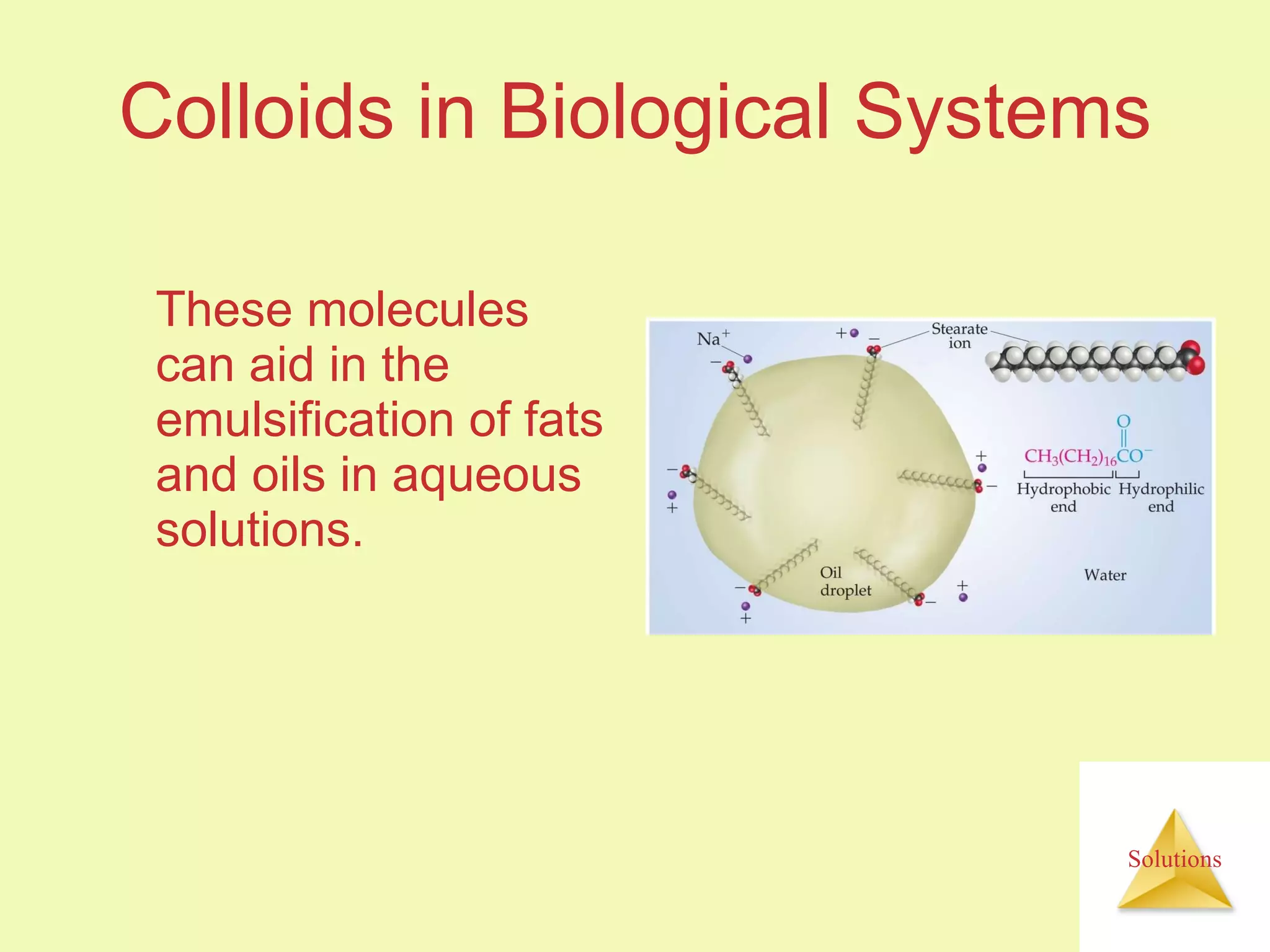
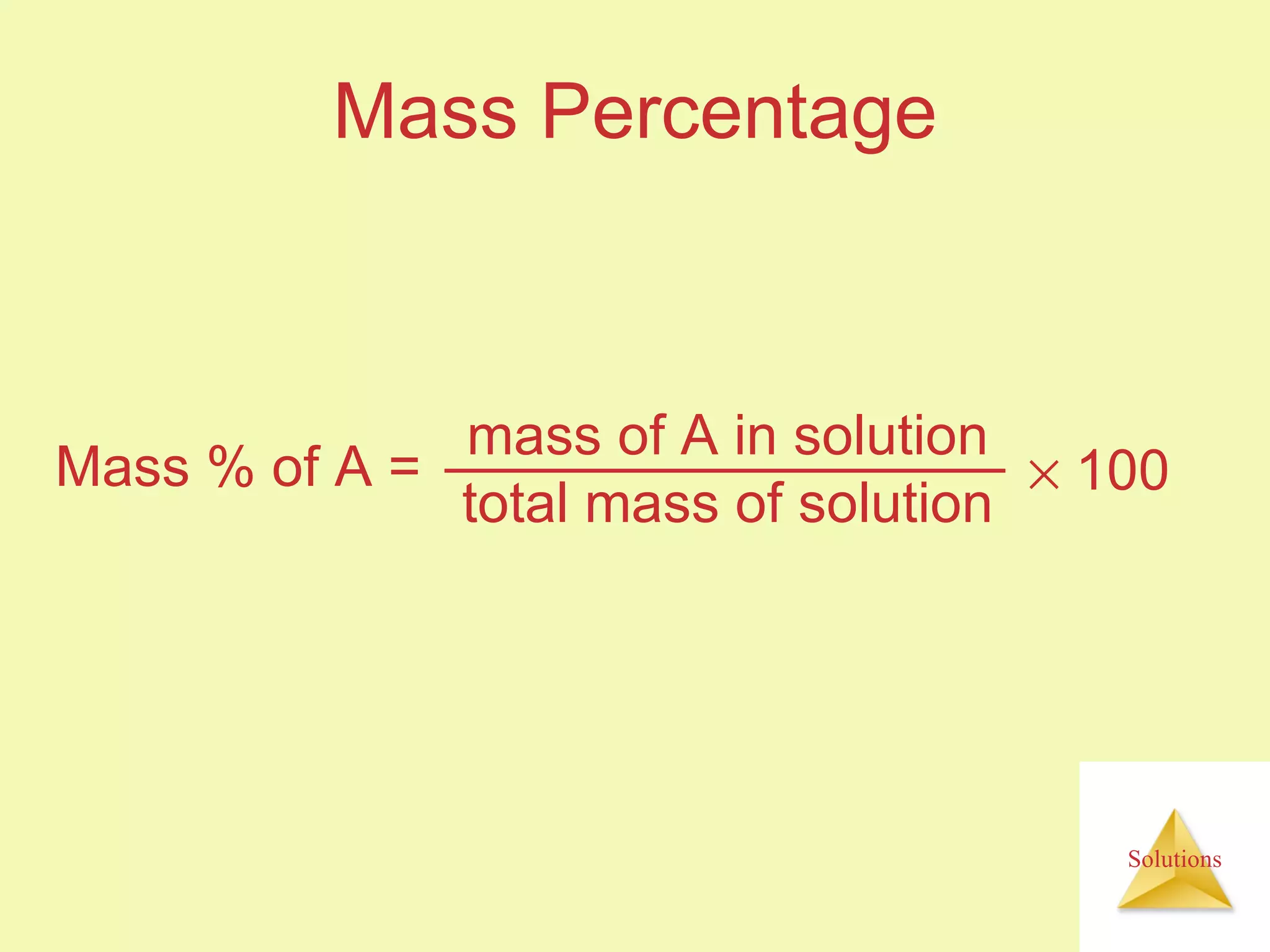This document discusses properties of solutions, including:
- Solutions are homogeneous mixtures of two or more substances, with the solute dispersed uniformly throughout the solvent.
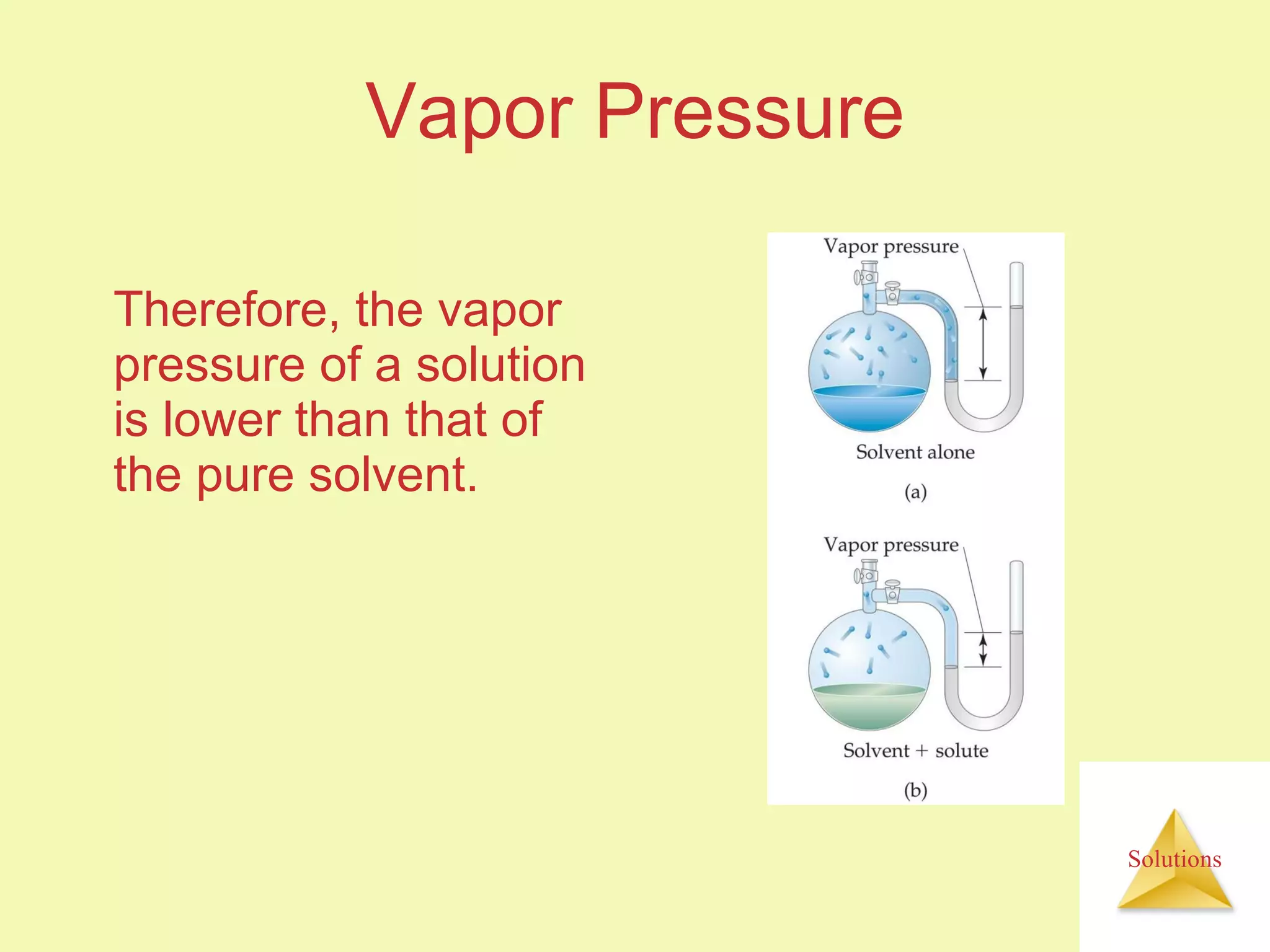
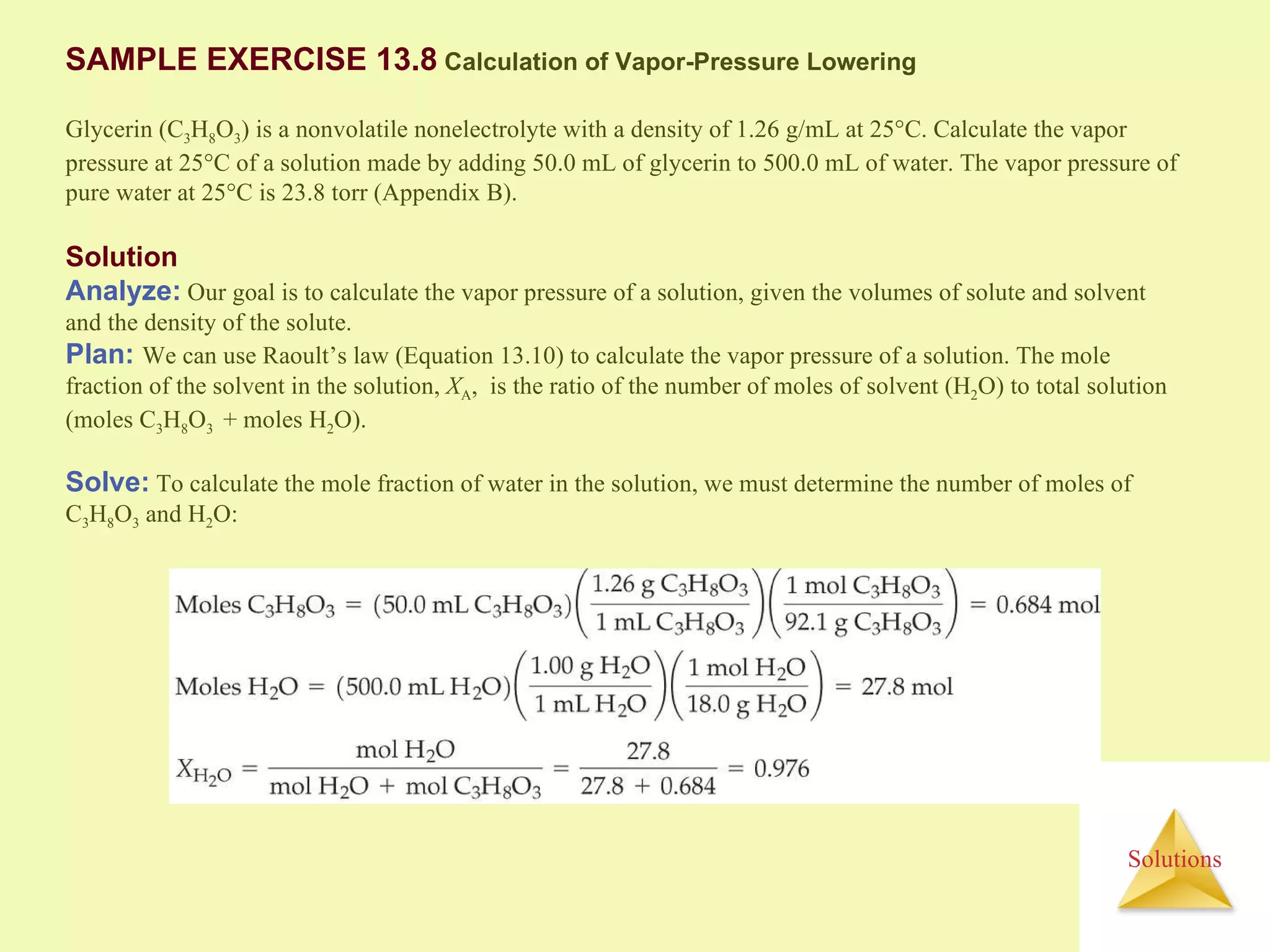
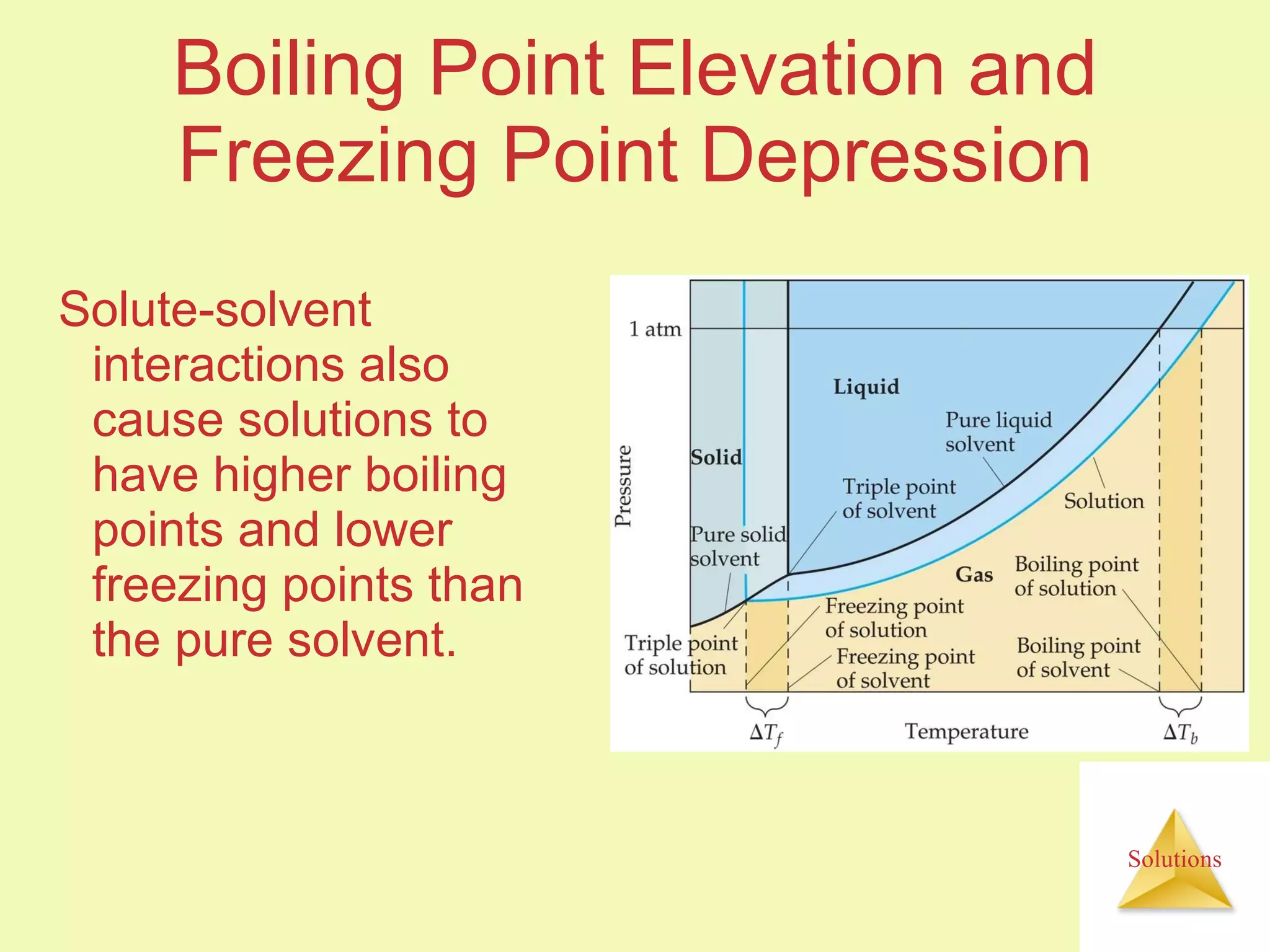
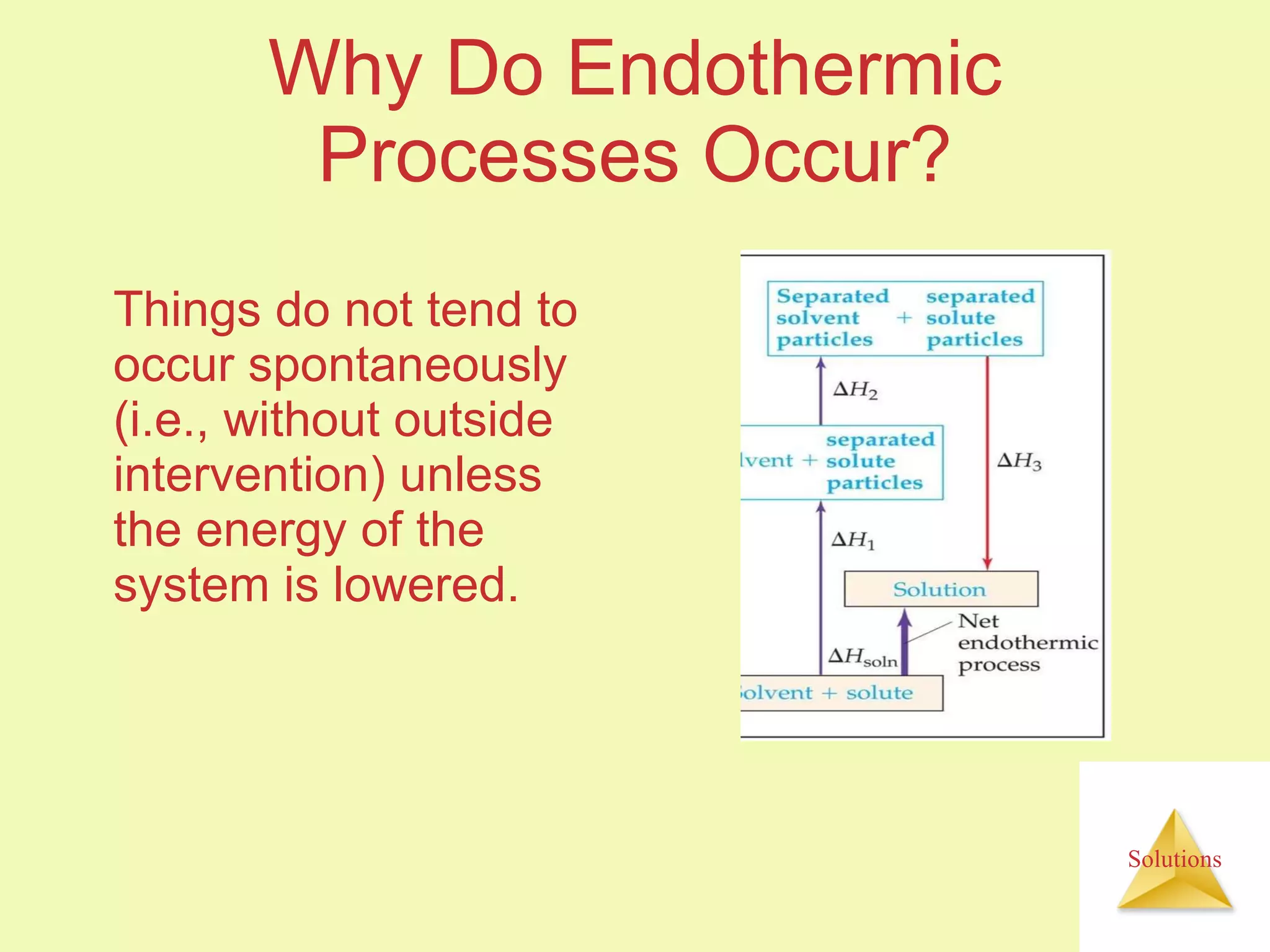
- A solute dissolves as the solvent molecules interact with and surround the solute particles or ions, changing the enthalpy of the system.
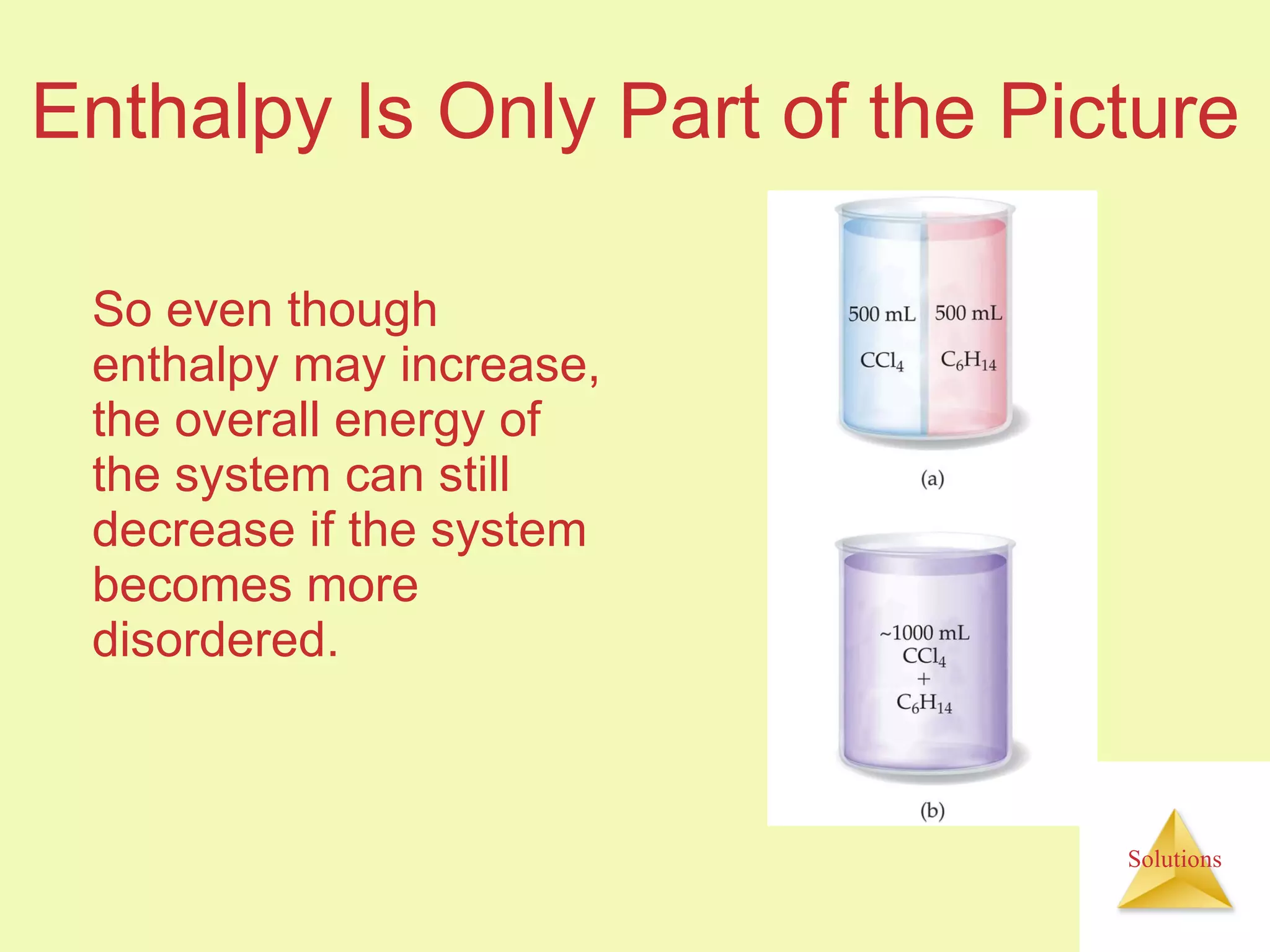
- The entropy of the system typically increases during dissolution, making dissolution spontaneous even for endothermic processes.
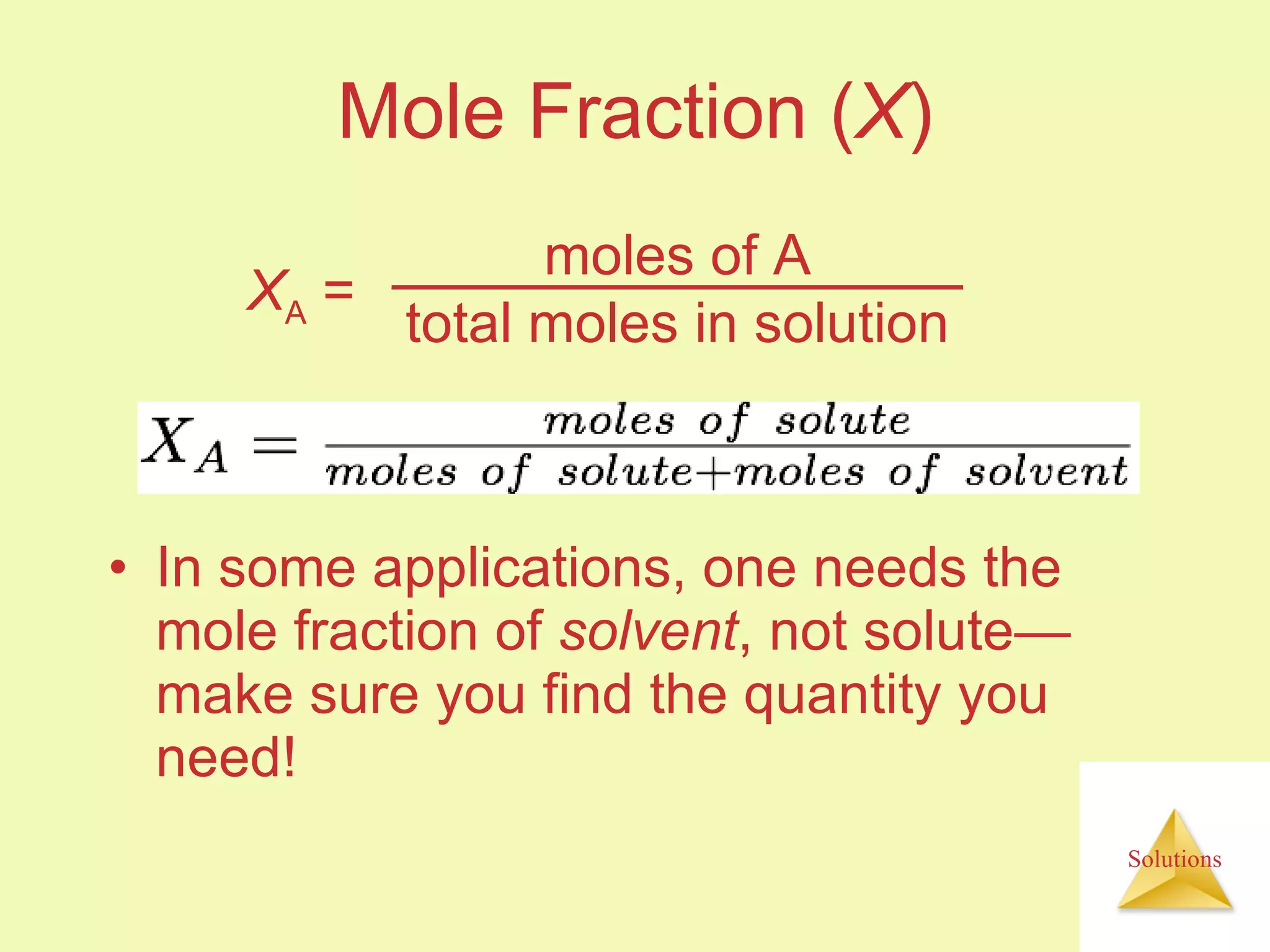
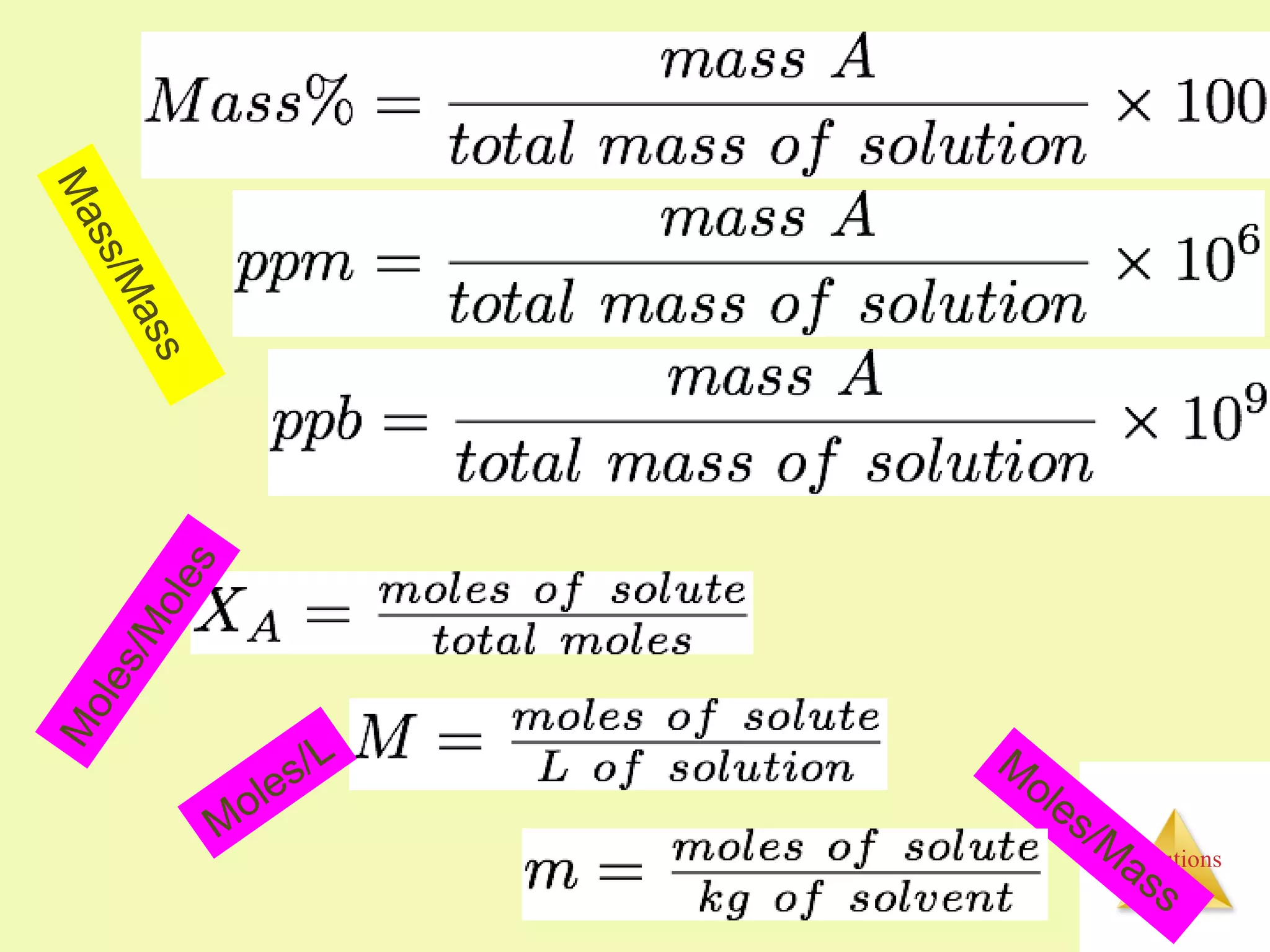
- Concentrations of solutions can be expressed using various units including molarity, molality, mass percent, and parts per million or billion.