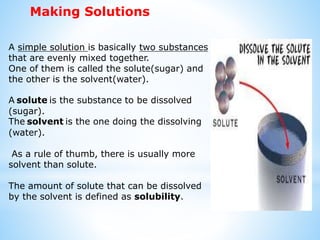
A solution is a homogeneous mixture composed of two or more substances. In a solution, the substance present in lesser amount is called the solute, which is dissolved in the substance present in larger amount known as the solvent. Common examples of solutions include sugar dissolved in water and gases dissolved in liquids. The concentration of a solute in a solution depends on how much of the solute is dissolved in the solvent. Solutions are homogeneous mixtures where the solute and solvent are mixed uniformly throughout the solution.