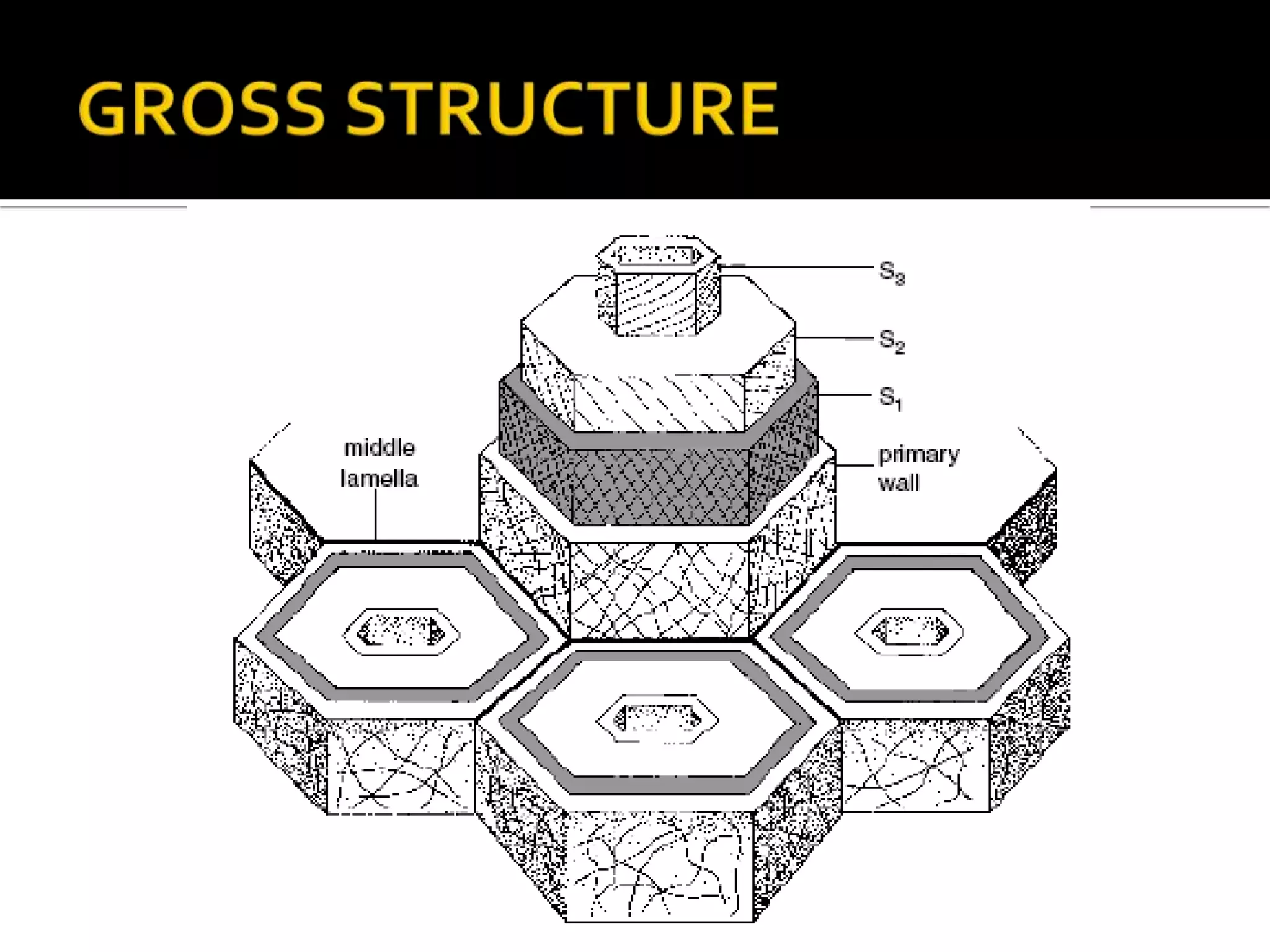
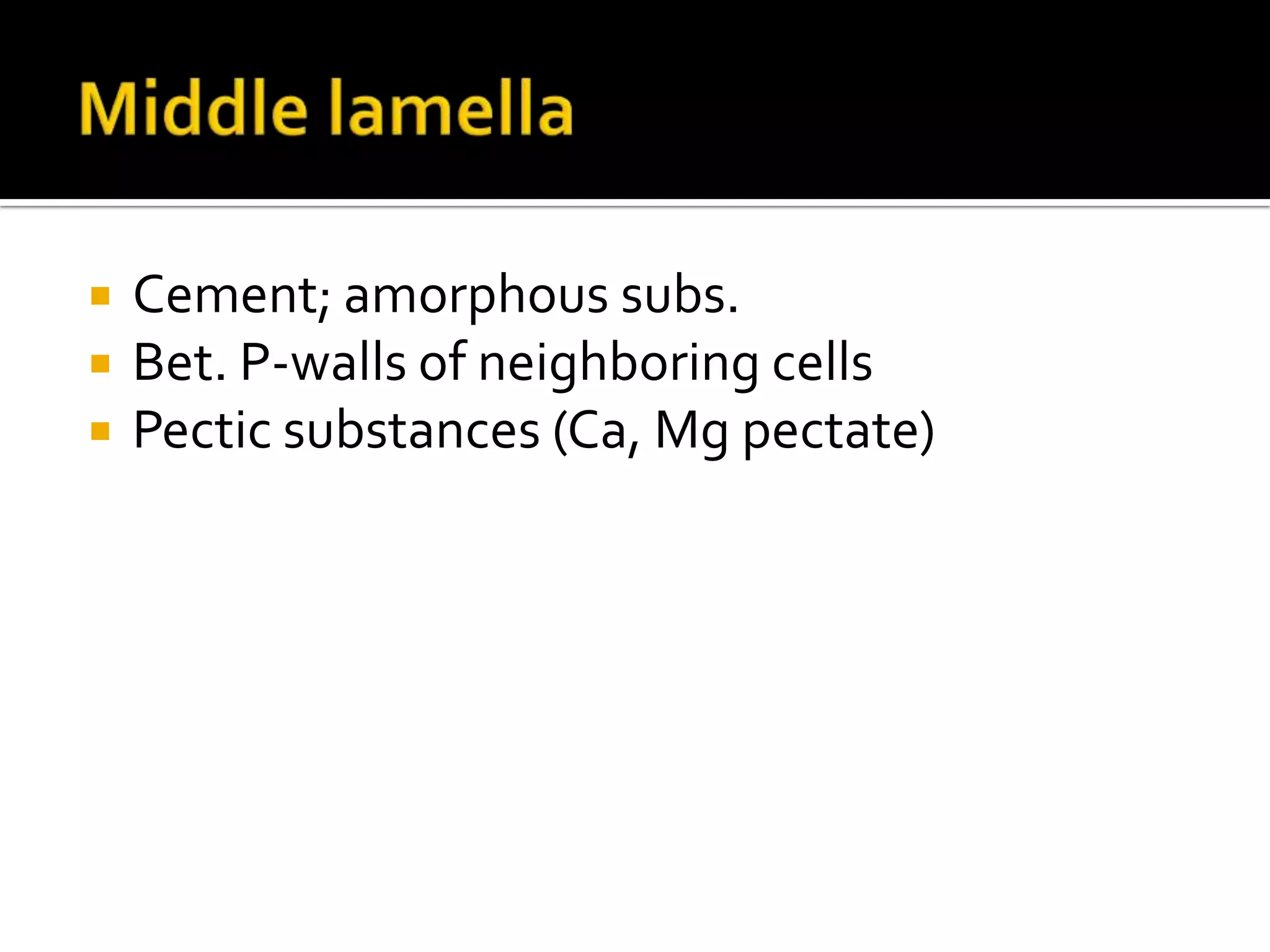
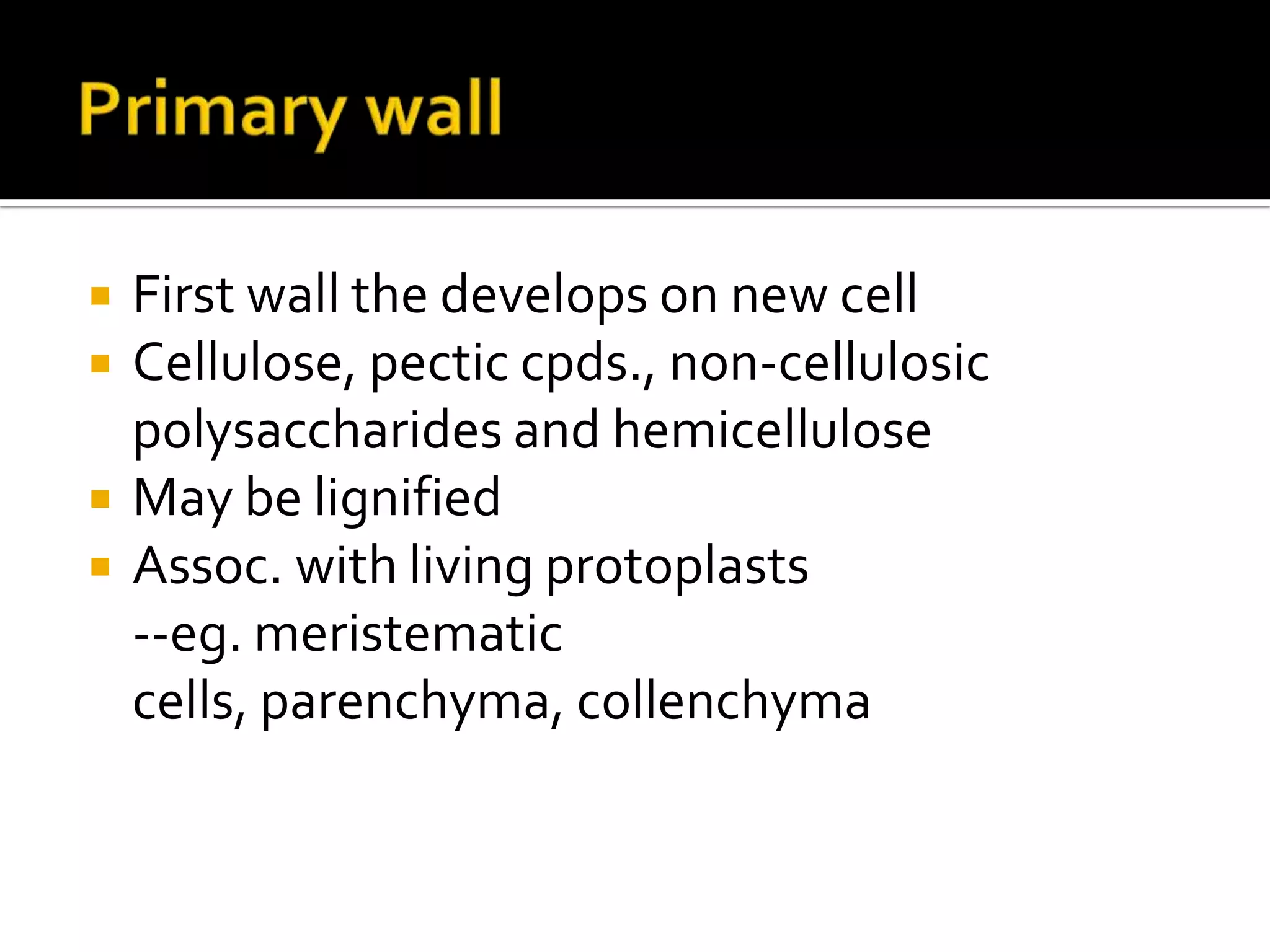
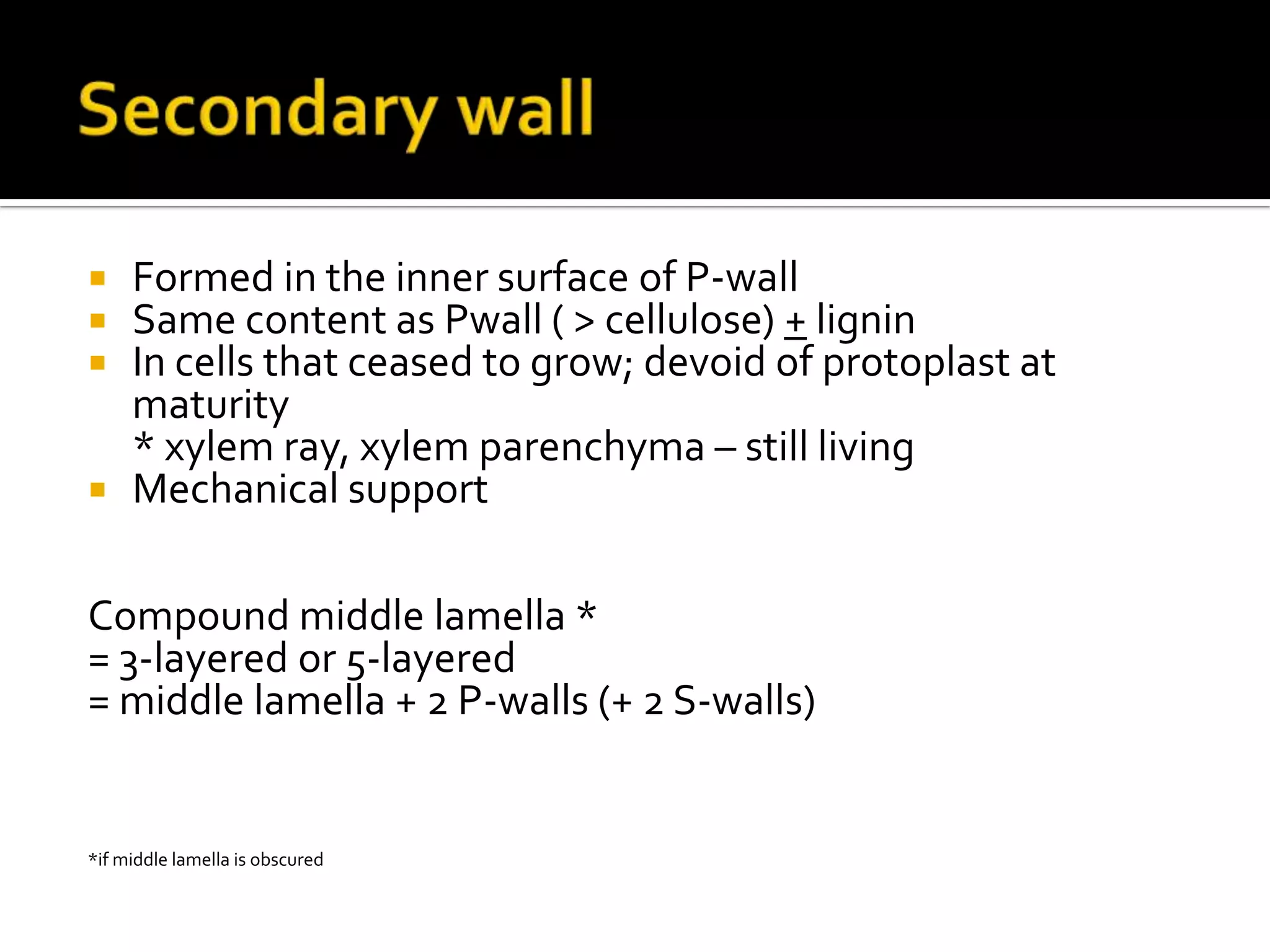
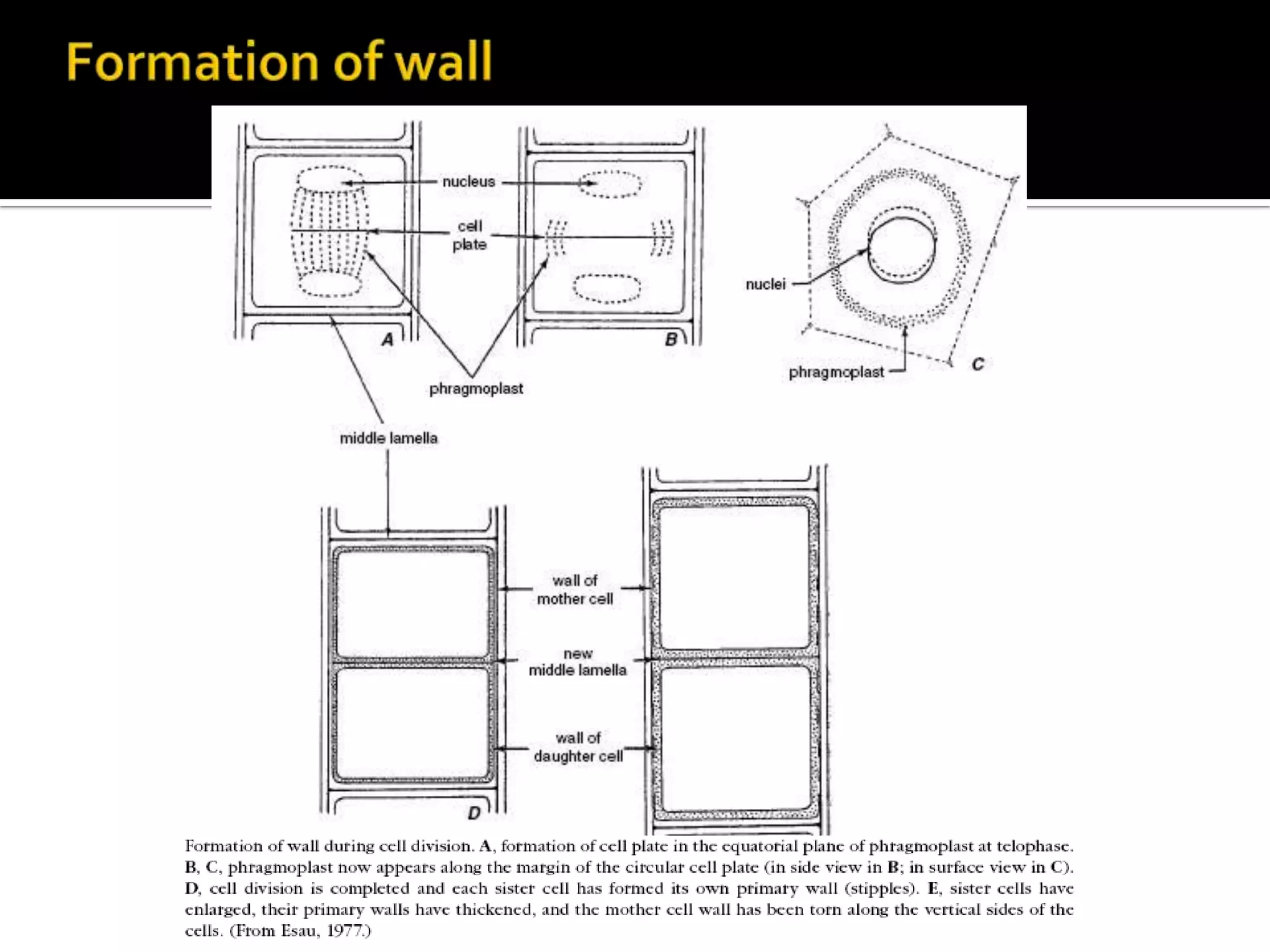
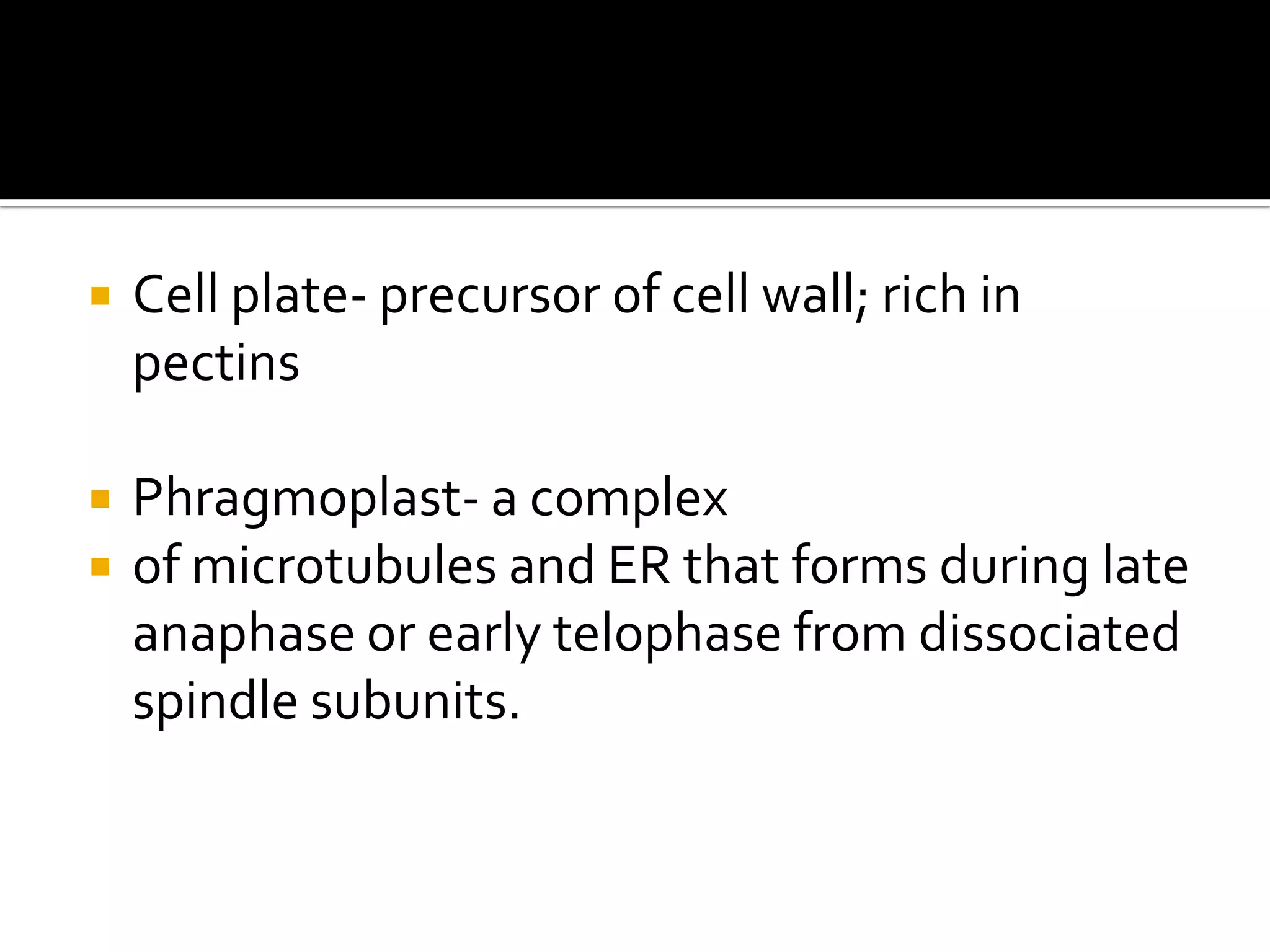
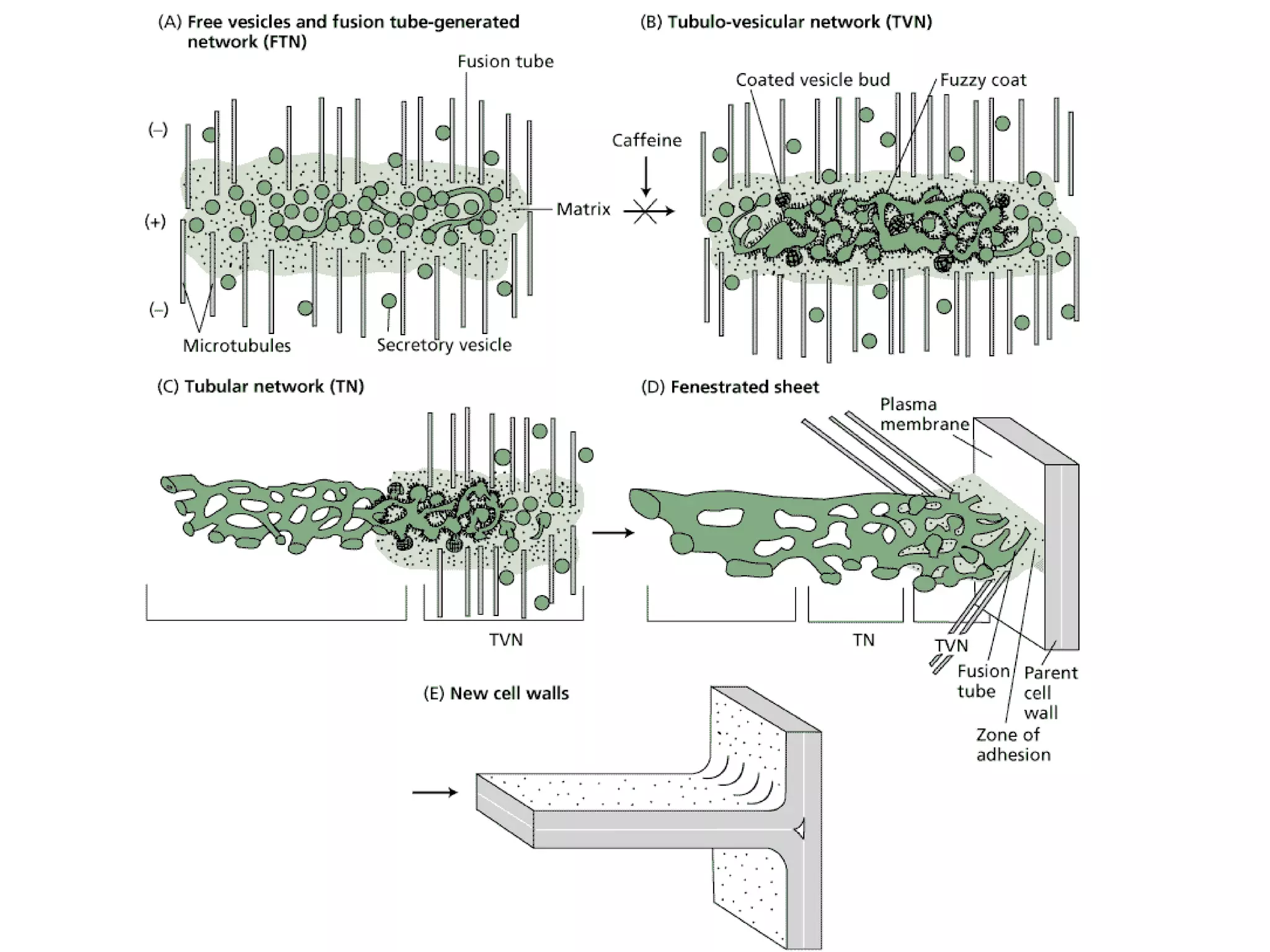
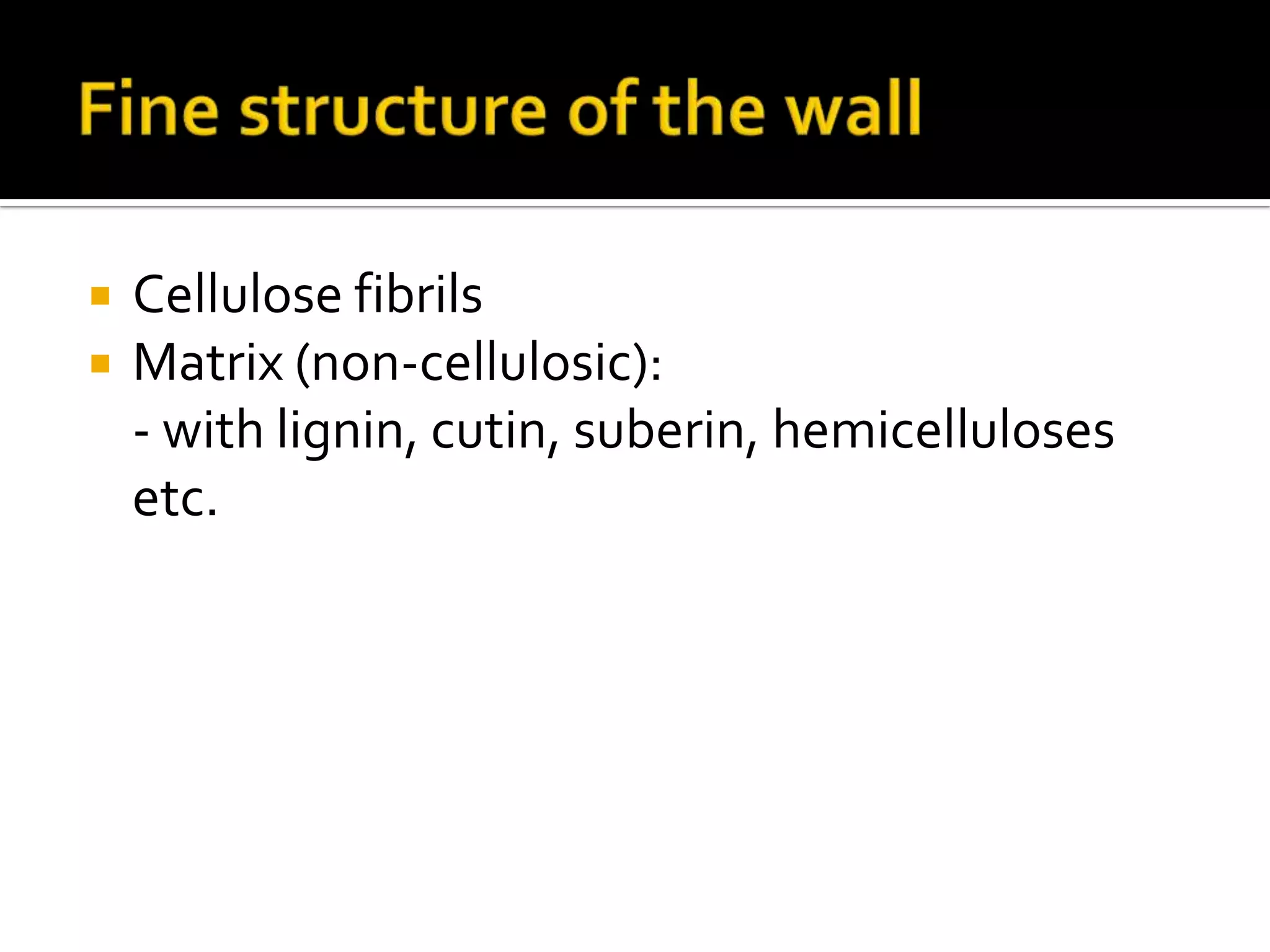
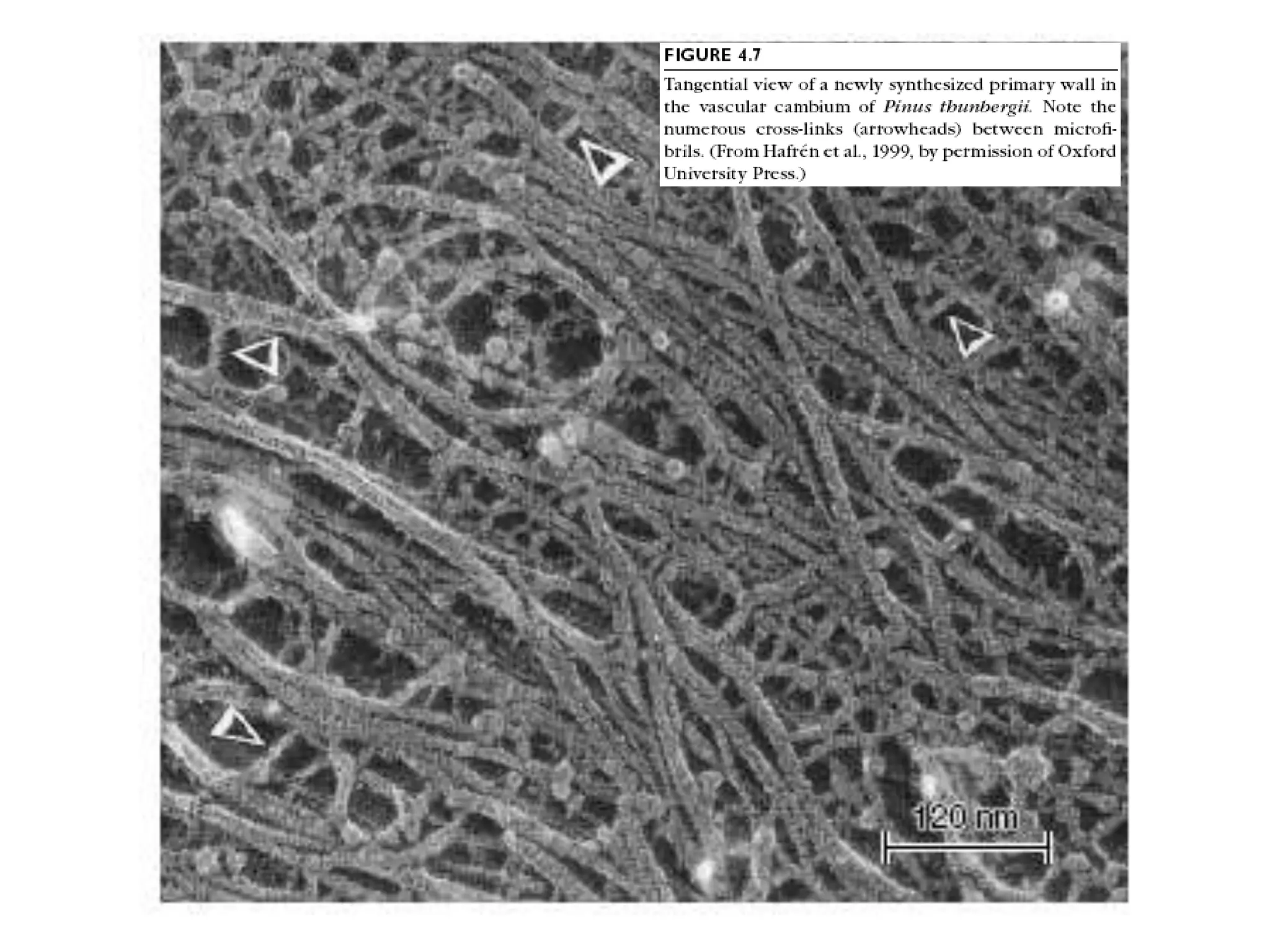
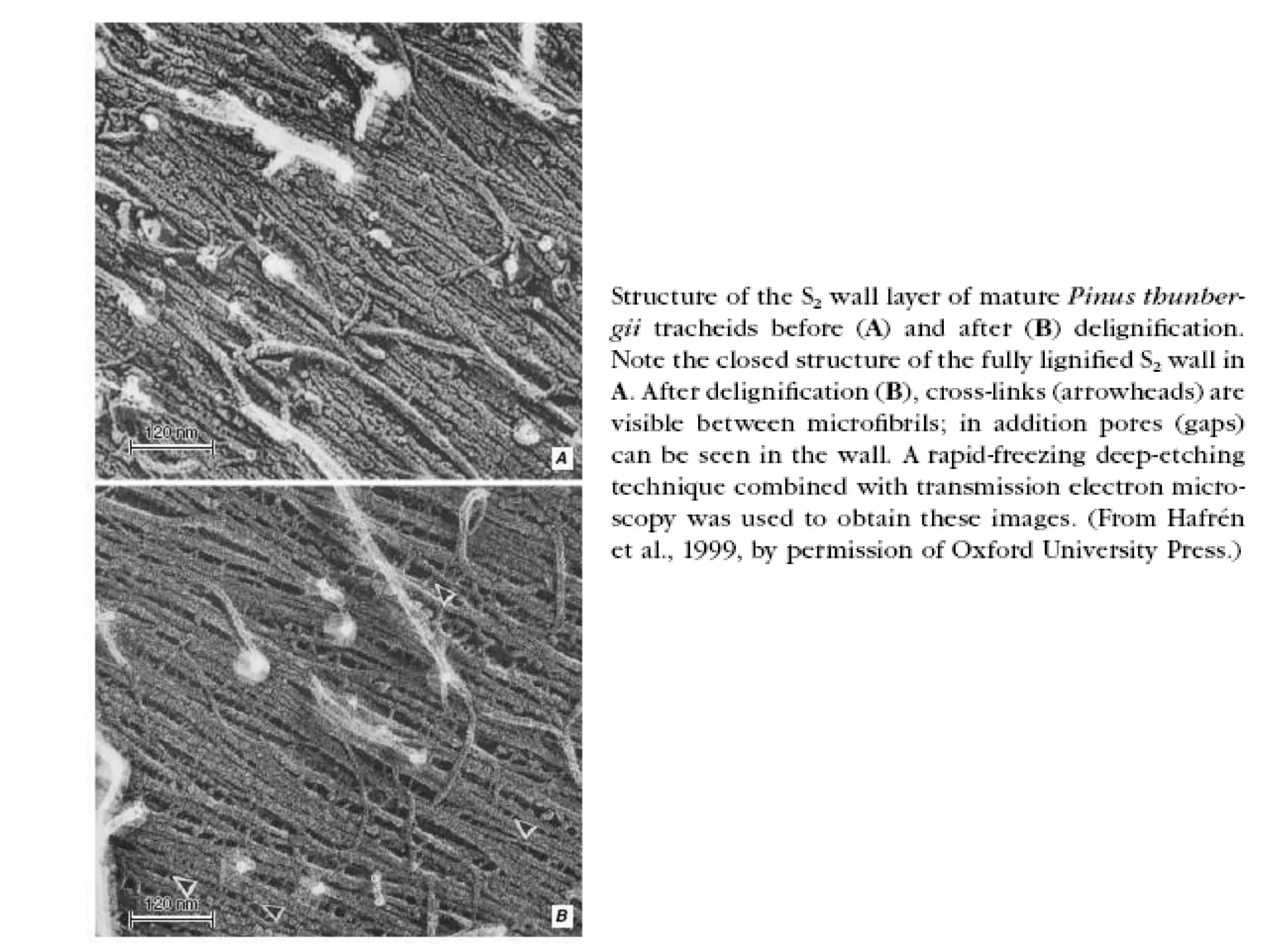
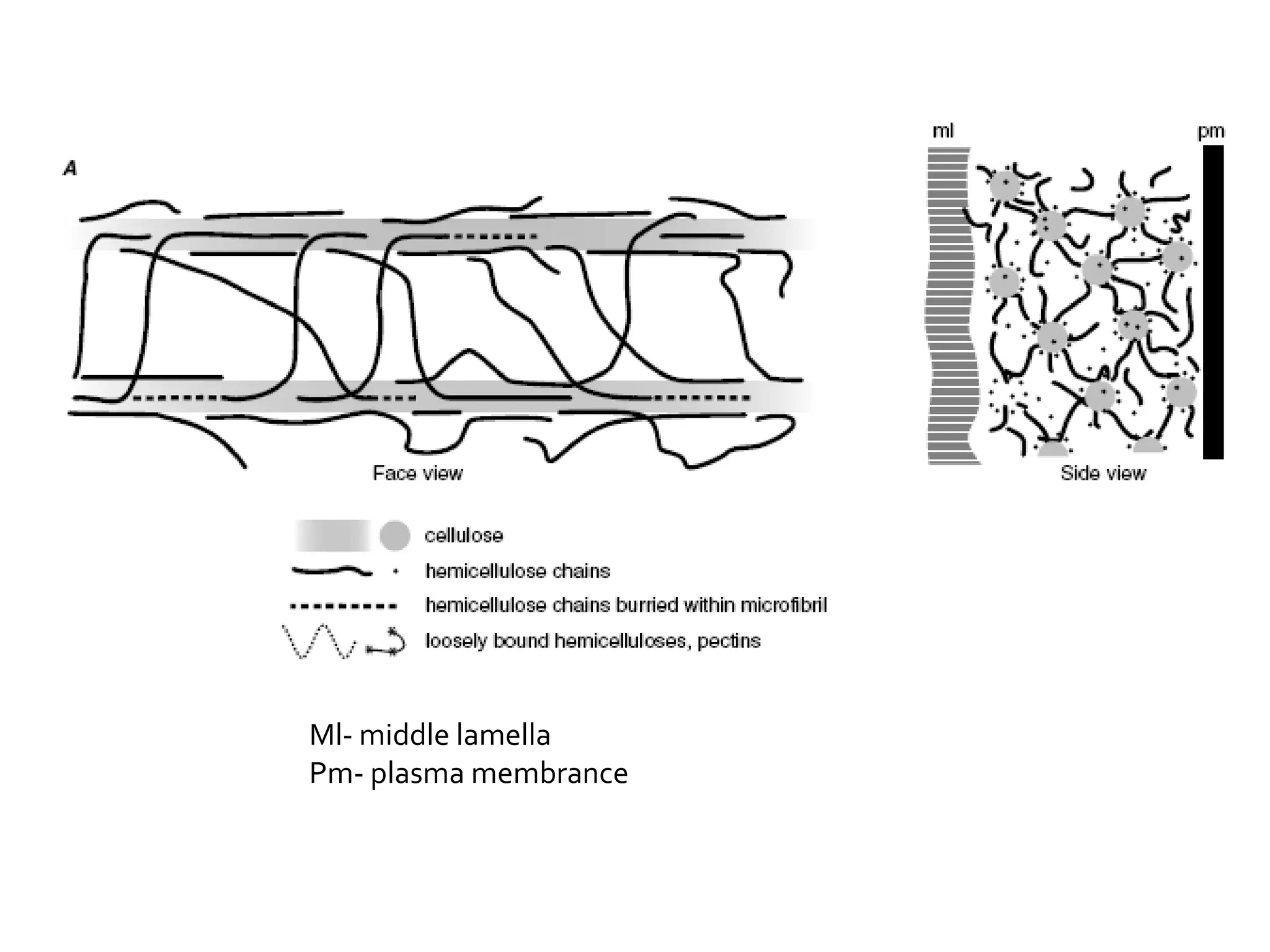
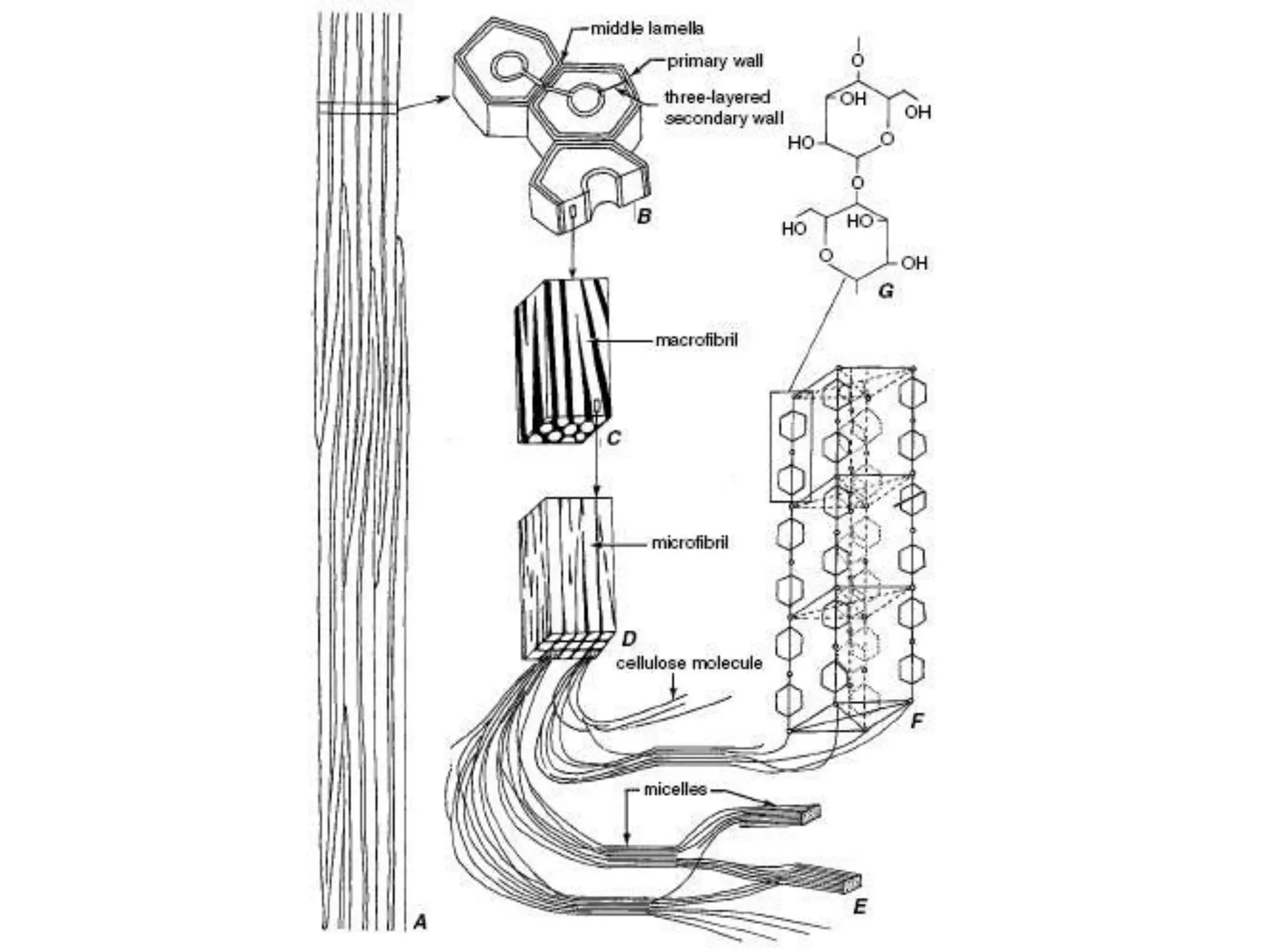
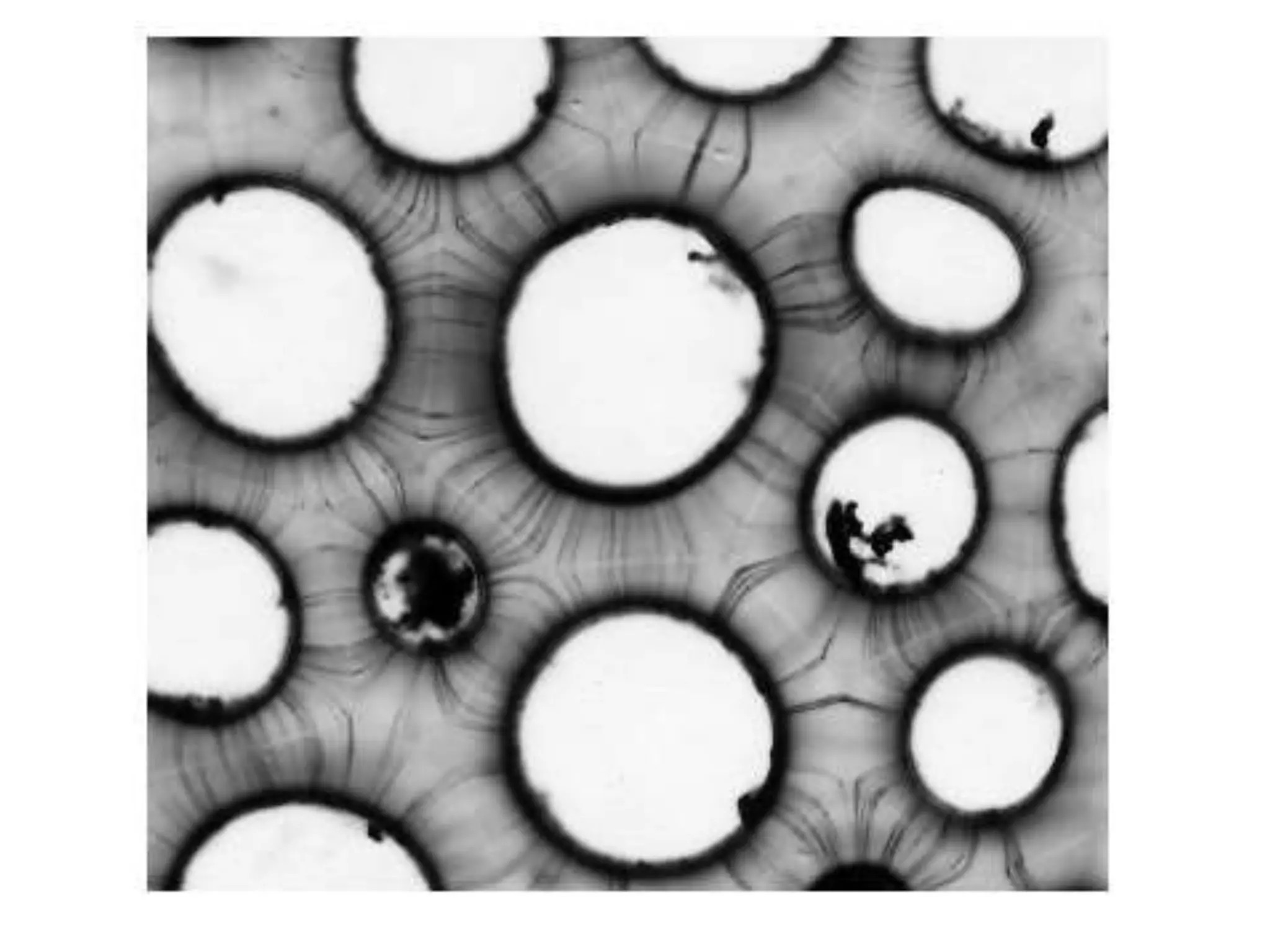
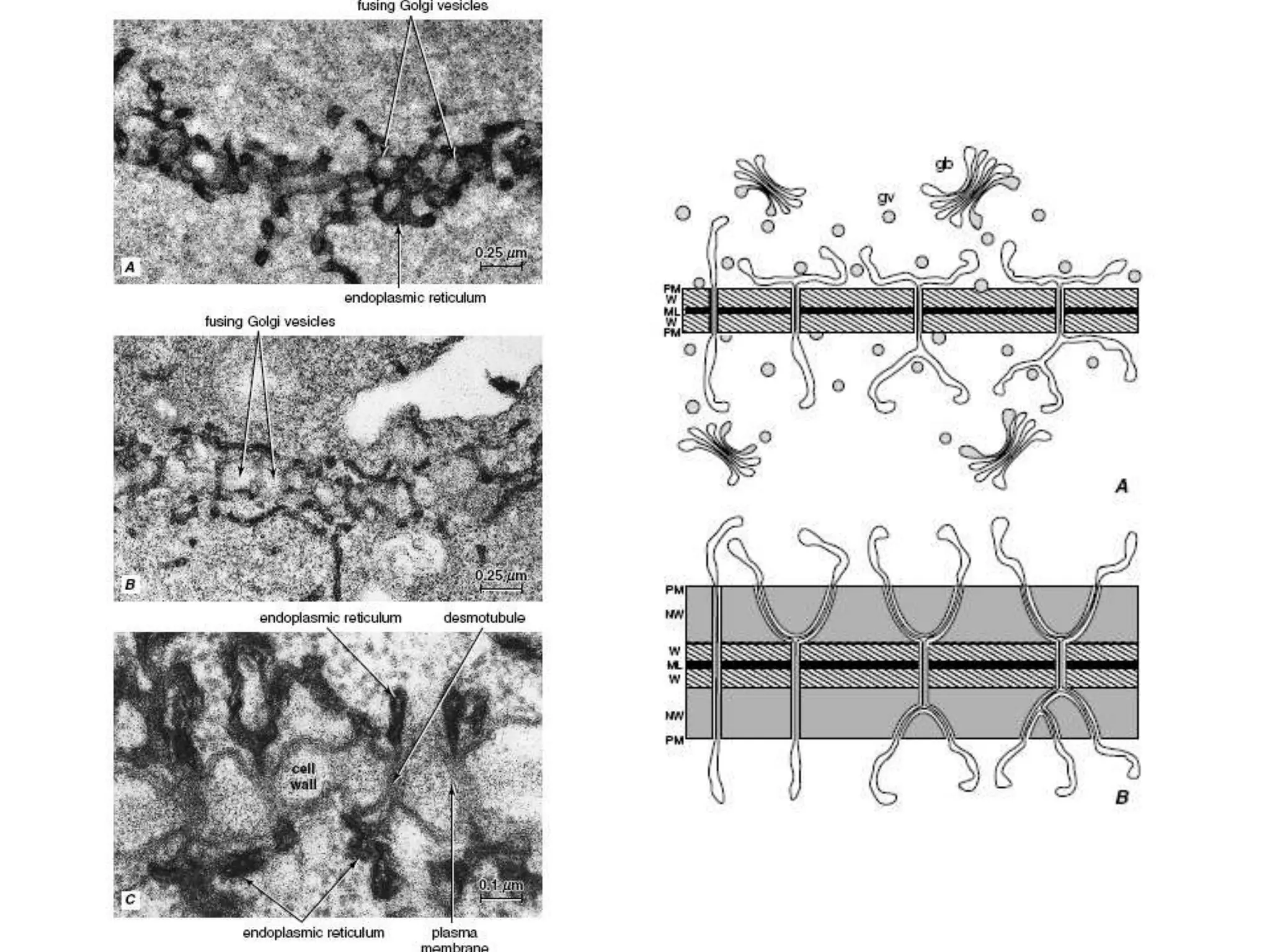
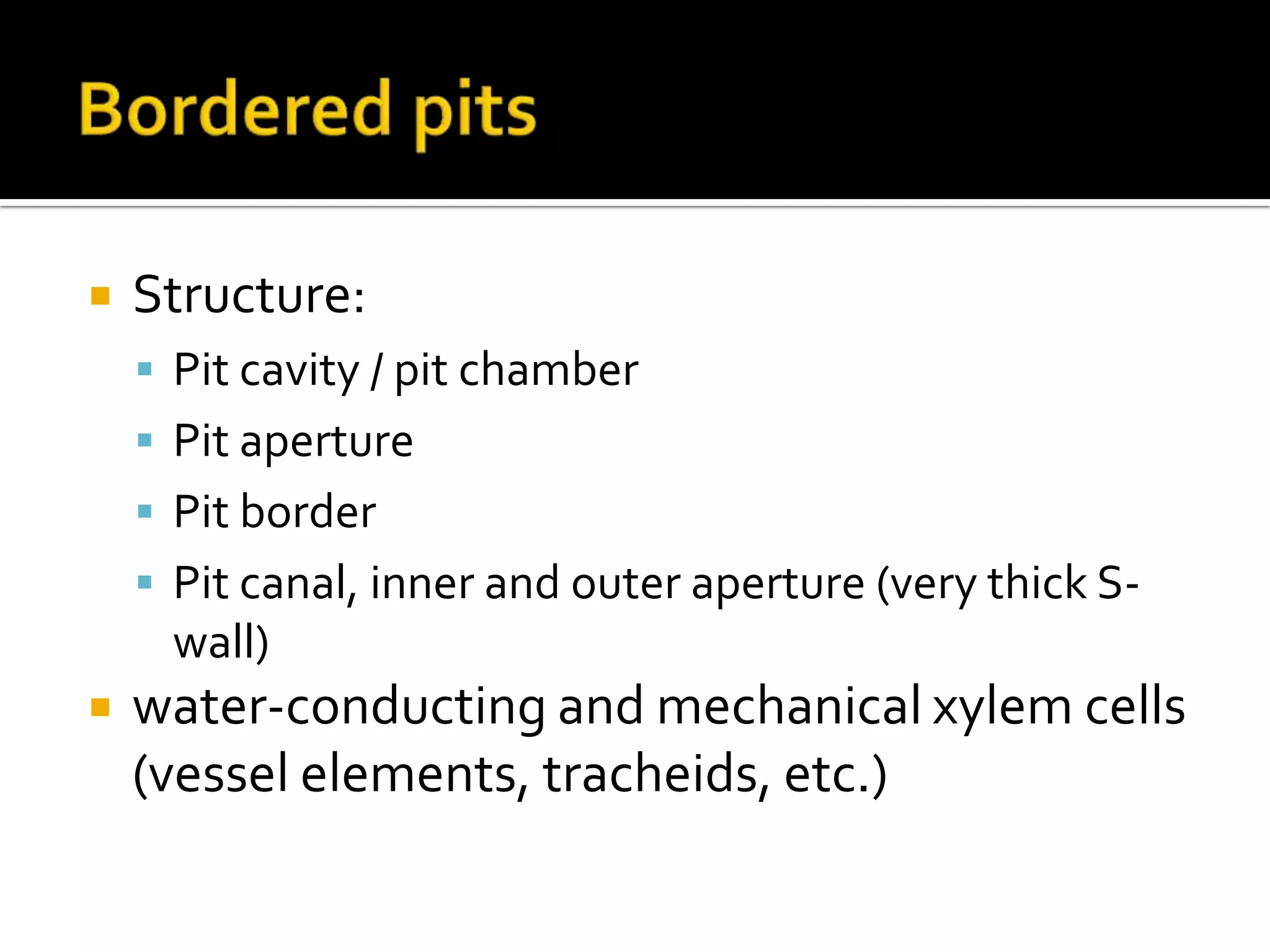
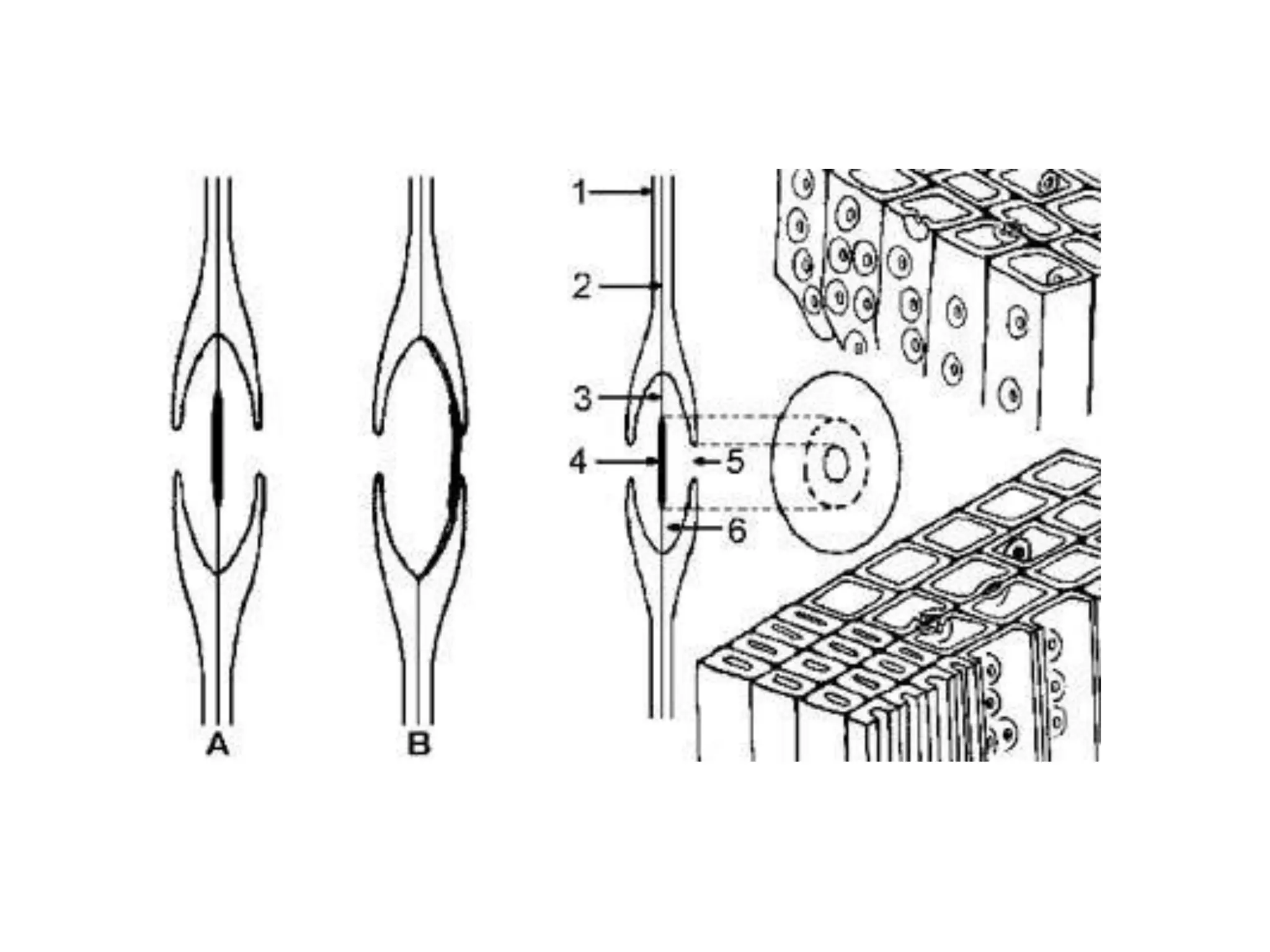
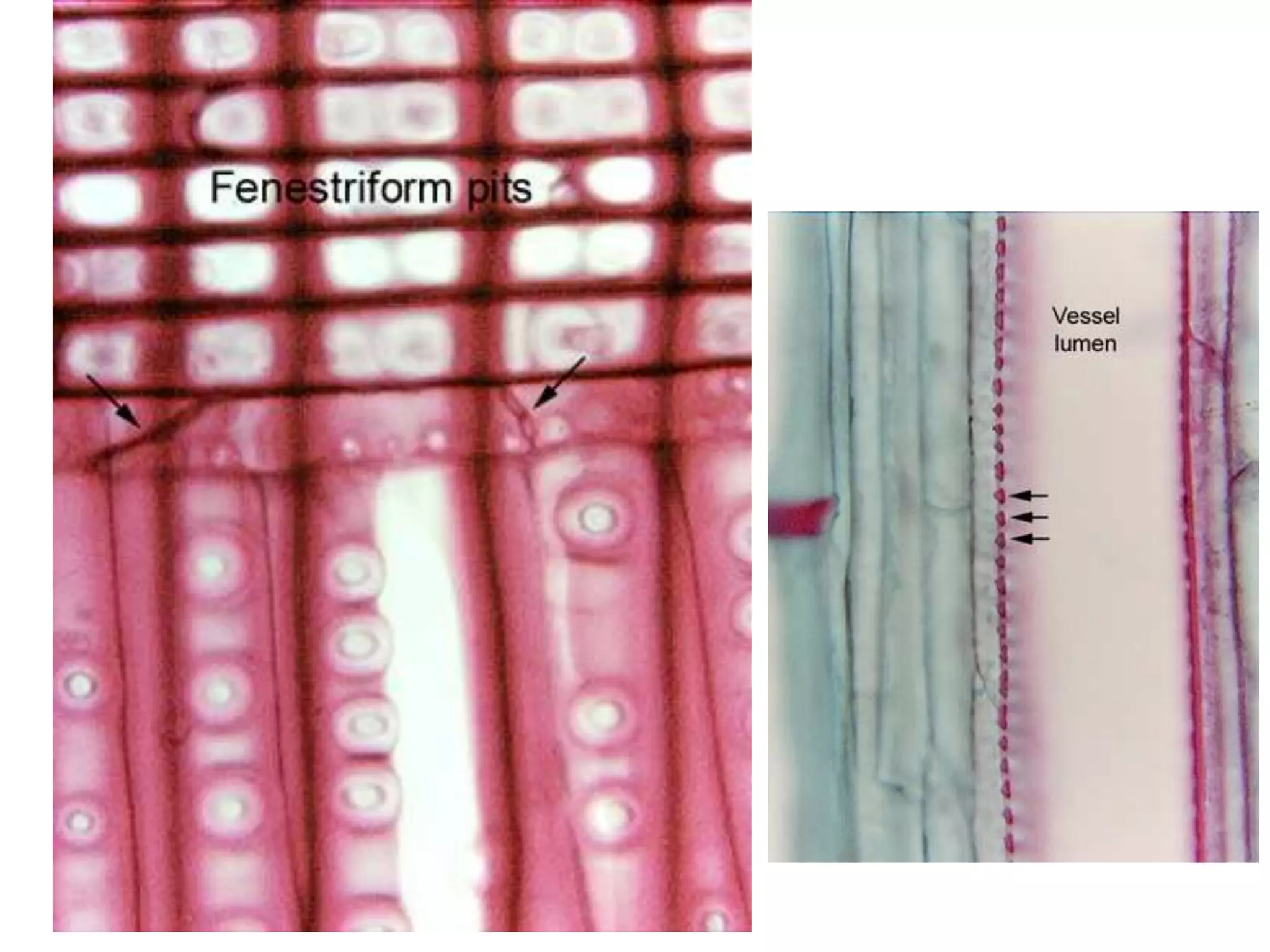
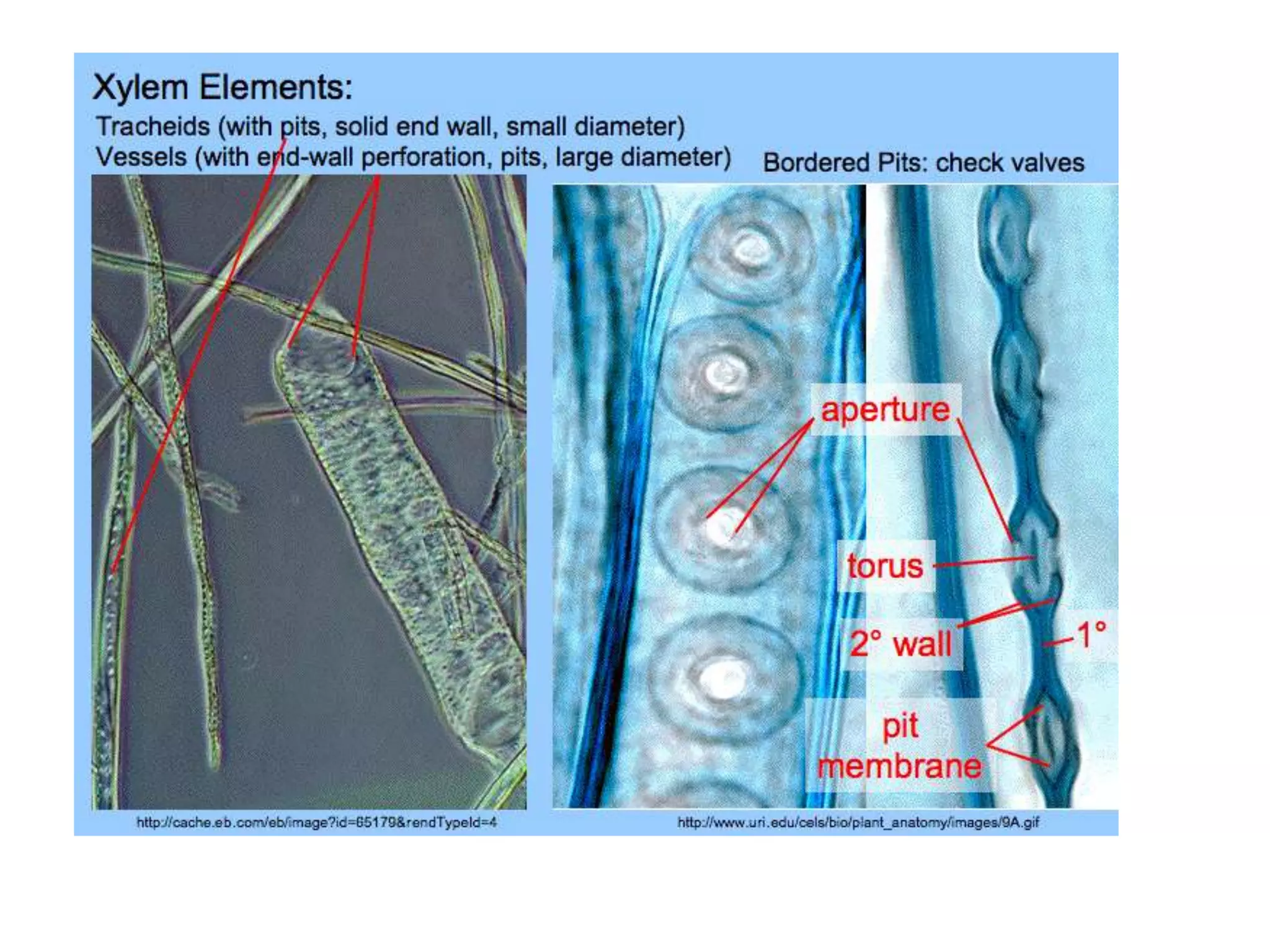
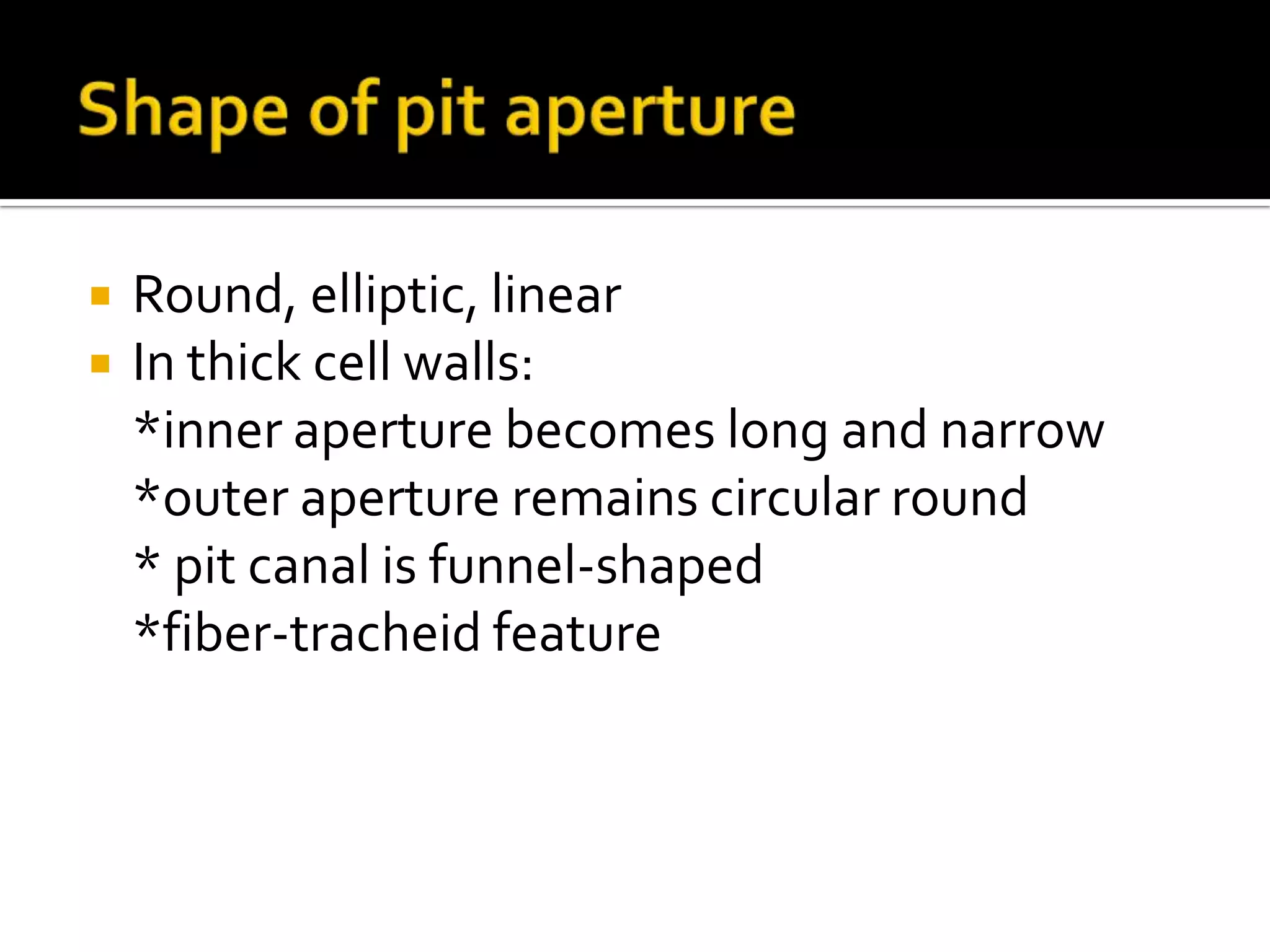
The cell wall provides structure and protection for plant cells. It has three main layers - the middle lamella, primary wall, and secondary wall. The middle lamella binds adjacent cells, the primary wall is the first layer deposited and allows cell growth, and the secondary wall provides support and is thicker with lignin. The cell wall is composed of cellulose microfibrils in a matrix of pectin and hemicellulose. It also contains structural components like lignin, cutin and suberin. Pit pairs and plasmodesmata allow communication between cells and transport through the cell wall. Bordered and simple pits are thin areas in the secondary wall that facilitate water and nutrient transport.