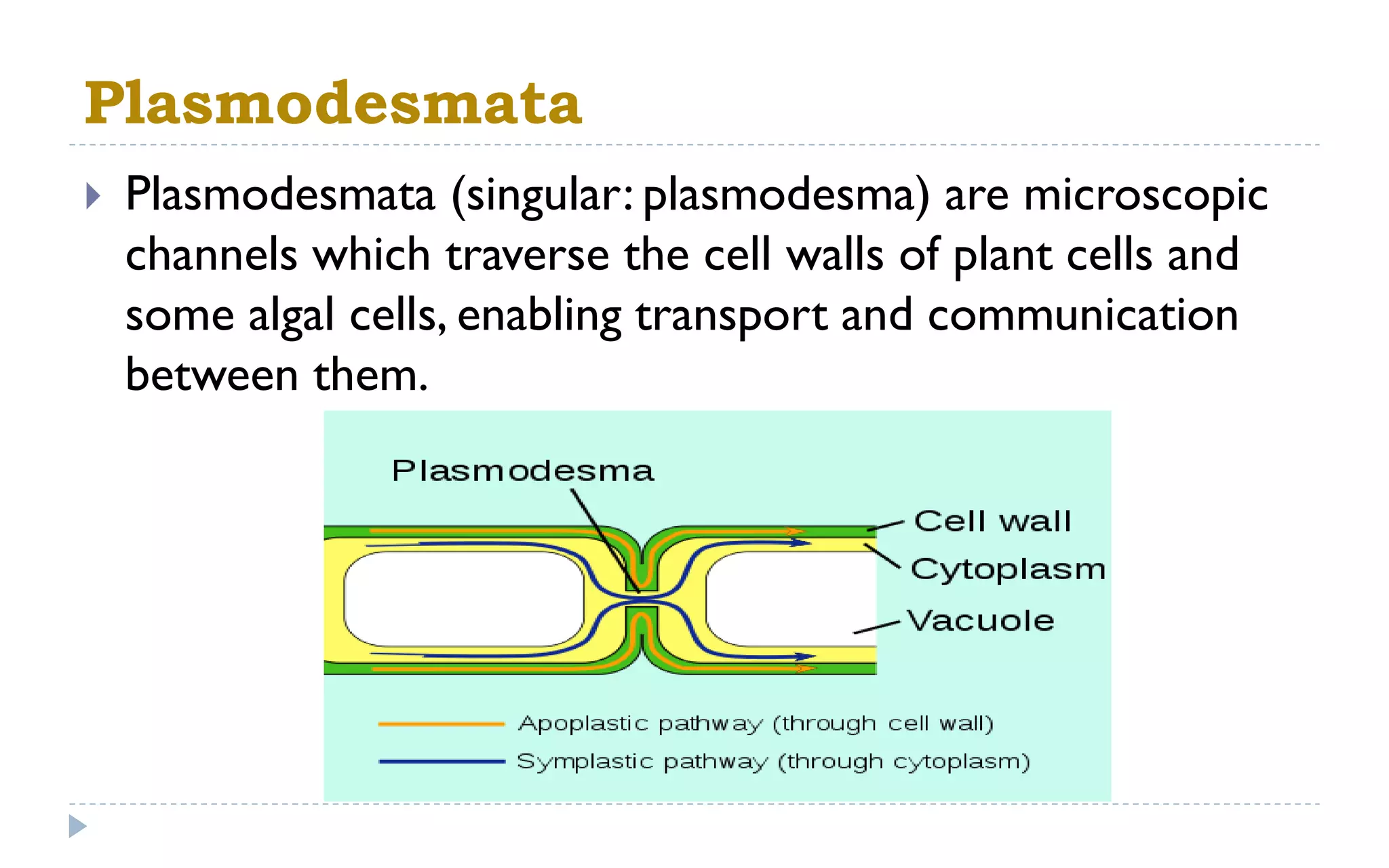
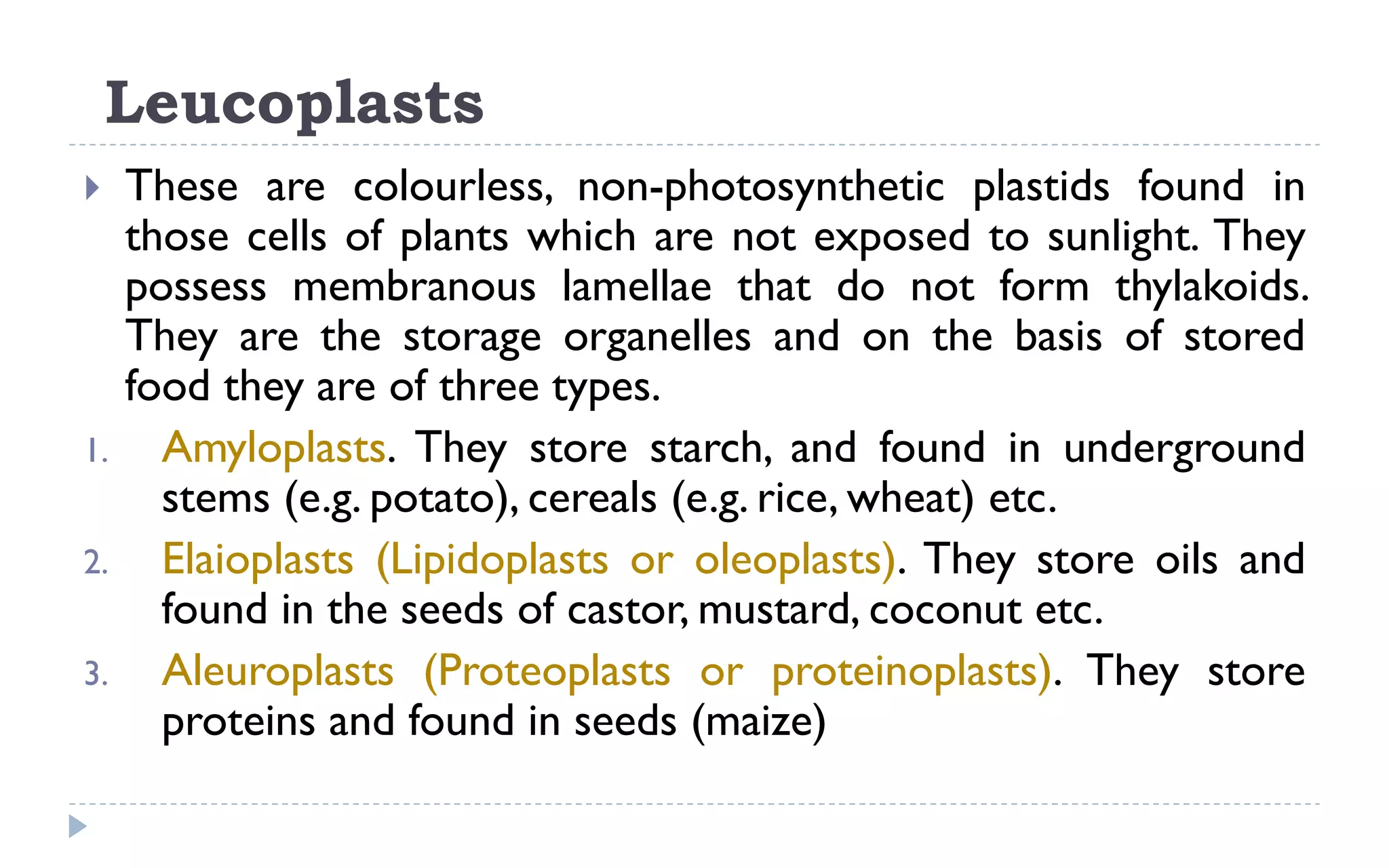
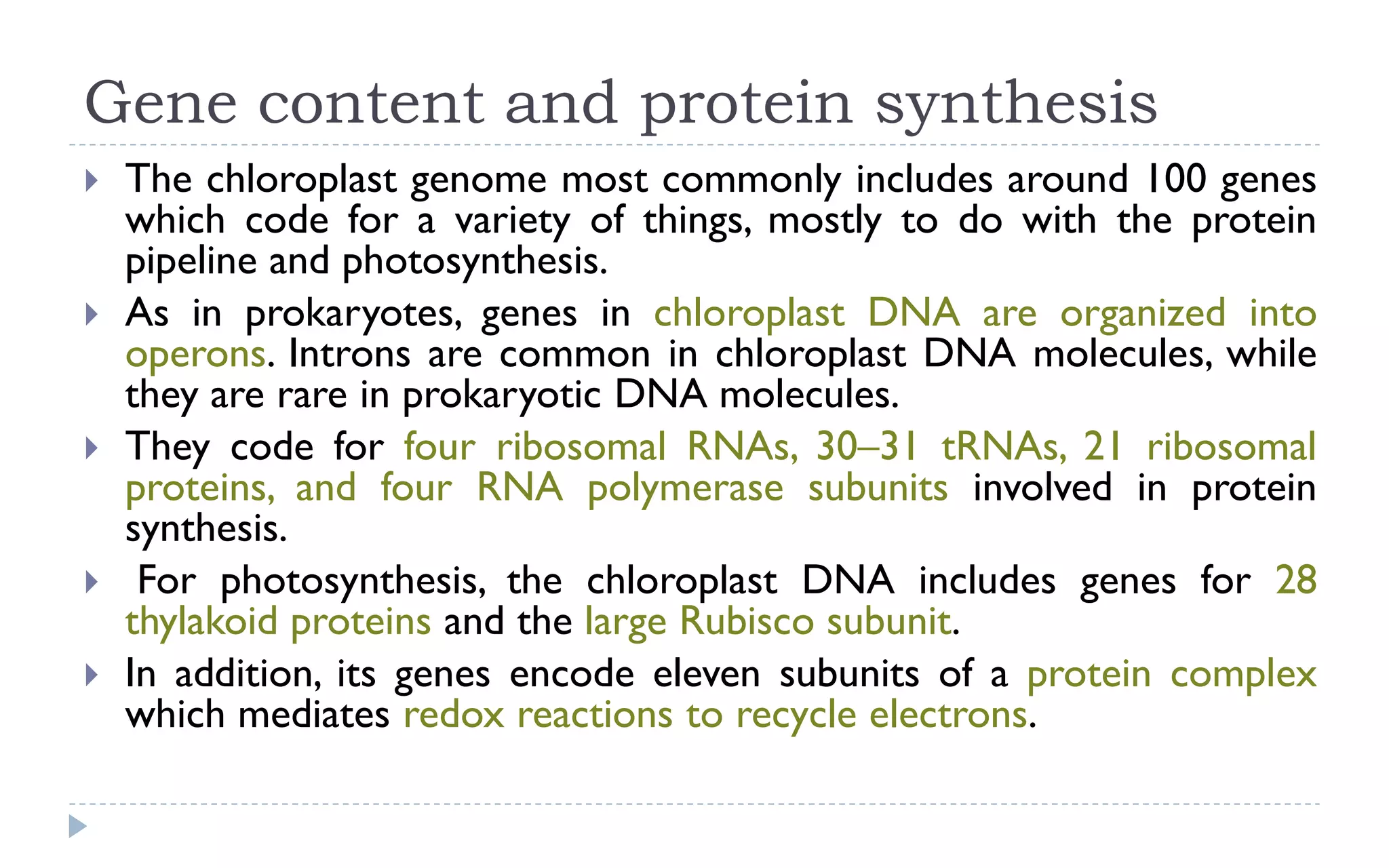
The document provides a detailed overview of plant cell apparatus, focusing on plasmodesmata and vacuoles. Plasmodesmata are microscopic channels enabling intercellular transport and communication, consisting of a desmotubule and cytoplasmic sleeve, while vacuoles serve as storage and regulatory compartments within plant cells, with significant roles in maintaining turgor pressure and isolating harmful substances. The document also discusses the formation, structure, function, and transport mechanisms associated with these organelles.