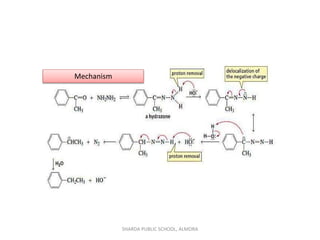
The document provides a comprehensive overview of aldehydes and ketones, detailing their structural characteristics, nomenclature, boiling points, solubility, and methods of preparation. It elaborates on their chemical properties, including reactions with nucleophiles, the formation of various derivatives, and specific reactions like the Cannizzaro reaction and aldol condensation. Additionally, it discusses the reactivity differences between aldehydes and ketones and their preparation methods, including oxidation and reduction processes.