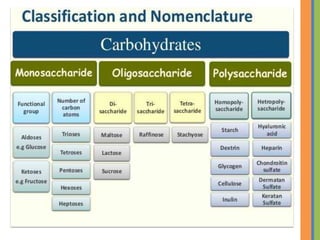
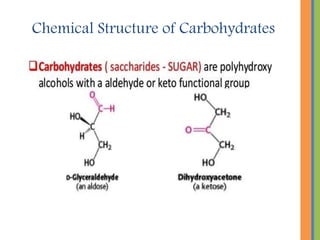
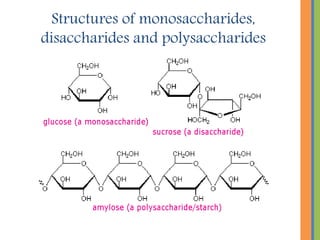
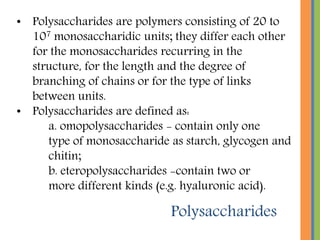
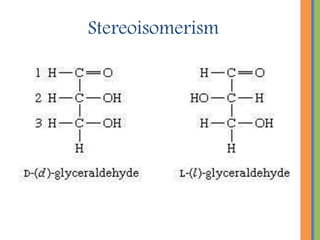
The document provides an overview of carbohydrates, detailing their chemical structure, classification into monosaccharides, disaccharides, and polysaccharides, along with their functions in the body as a primary energy source. Carbohydrates, stemming from photosynthesis, are essential organic molecules found in many foods and include examples like glucose, sucrose, and starch. The document also discusses the importance of stereoisomerism in the structural variations of carbohydrates.