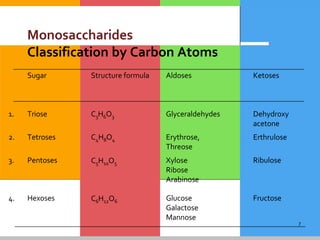
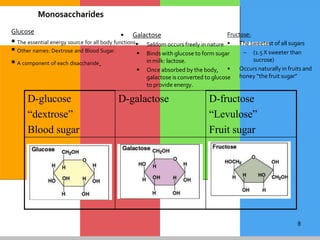
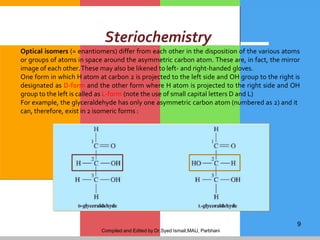
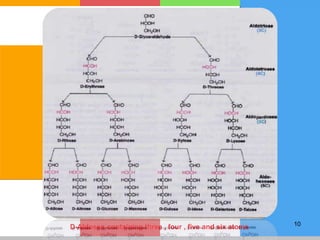
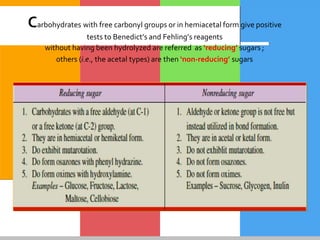
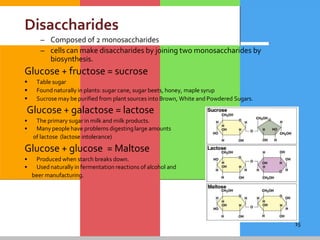
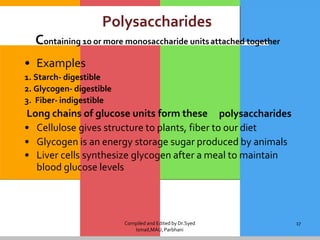
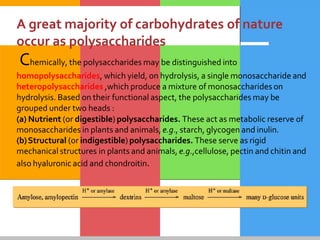
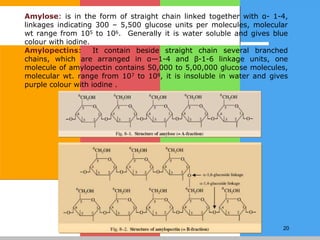
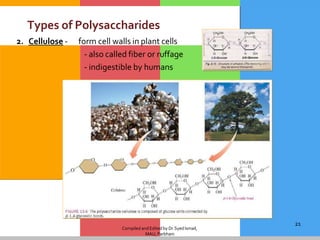
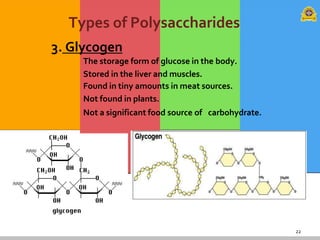
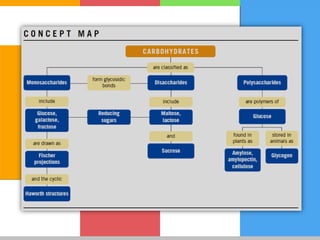
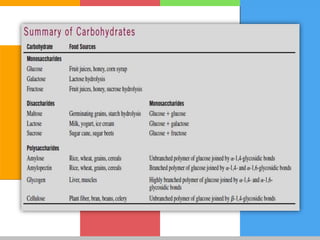
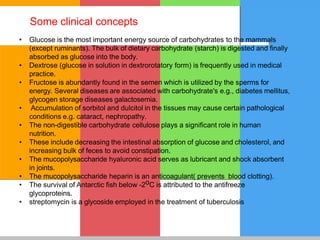
The document provides a comprehensive overview of carbohydrates, describing their structure, classification, and functions in living organisms. It explains the types of carbohydrates including monosaccharides, disaccharides, oligosaccharides, and polysaccharides, along with their roles in energy storage and cellular structure. It also discusses the significance of carbohydrates in human nutrition, their metabolic processes, and associated health concerns.