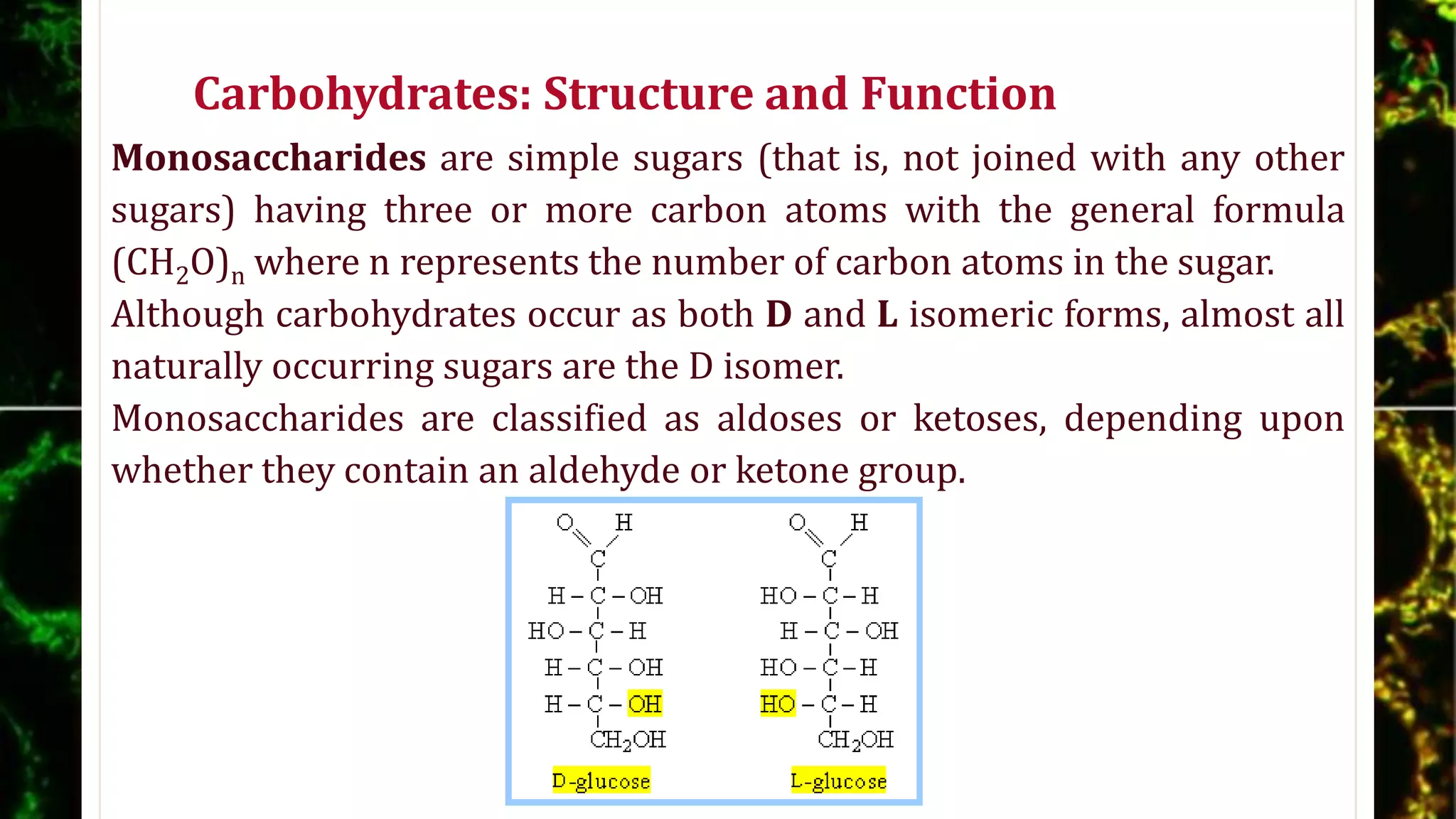
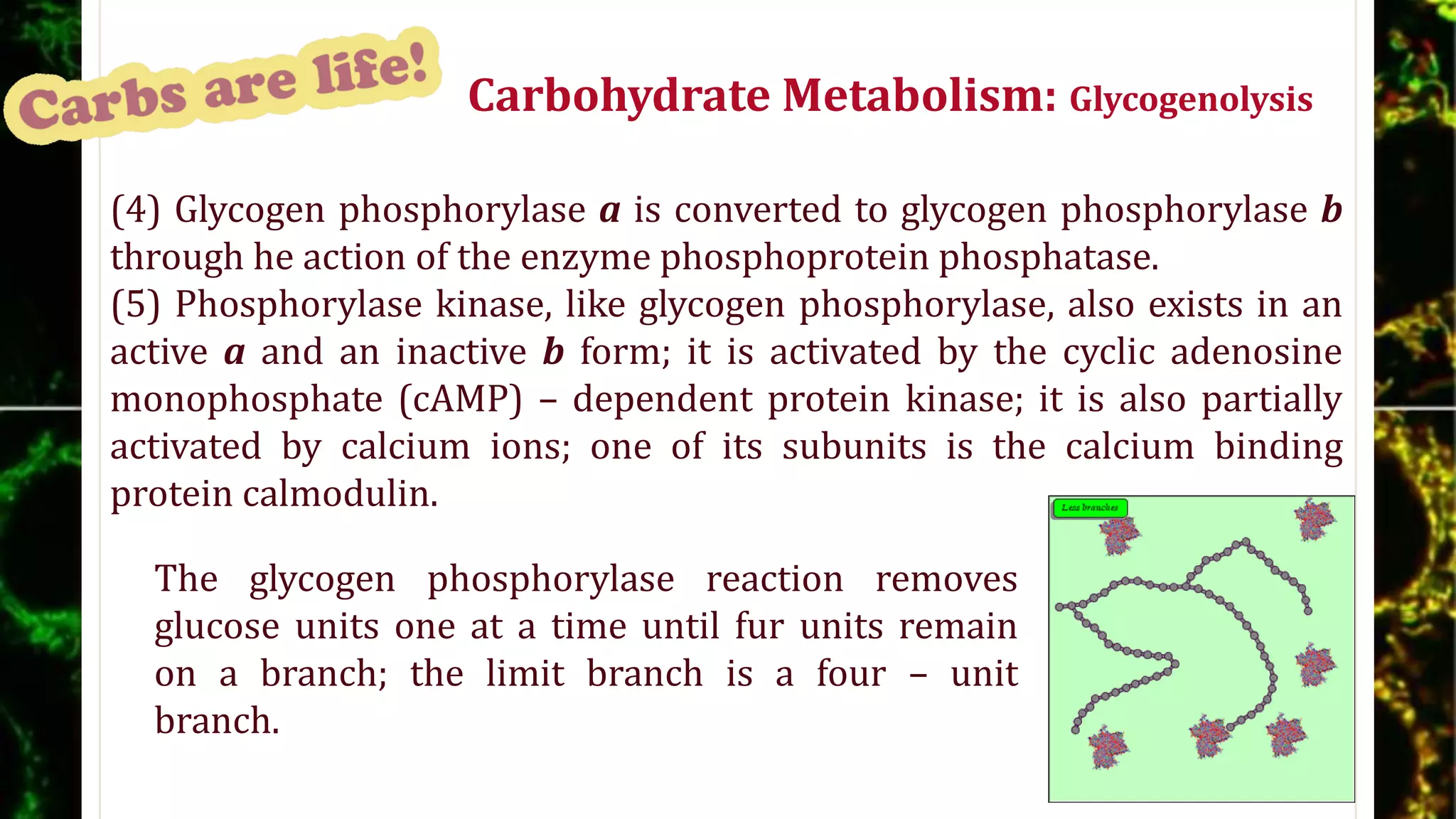
The document discusses carbohydrates, detailing their structures, functions, and metabolic processes, emphasizing their role as energy sources, storage forms, and structural components in living organisms. It explains the classification of carbohydrates into monosaccharides, disaccharides, oligosaccharides, and polysaccharides, along with the biochemical reactions involved in their synthesis and degradation, particularly glycogenolysis and glycogenesis. Additionally, the document highlights the hormonal regulation of carbohydrate metabolism and the pentose phosphate pathway's function in producing reducing equivalents and ribose-5-phosphate.

































![Carbohydrate Metabolism:
The Pentose Phosphate Pathway
The pentose phosphate pathway, also called
the hexose monophosphate shunt or the
phosphogluconate pathway, has two
functions: to generate reducing equivalents
and produce ribose 5 – phosphate.
Many biochemical reactions consume energy;
biochemical systems supply this energy
through molecules that carry reducing
equivalents (for example NADH and NADPH)
and through molecules that carry high-
energy phosphate bonds ( for example, ATP,
guanosine triphosphate [GTP], and UTP).](https://image.slidesharecdn.com/carbohydrates-210610150113/75/Carbohydrates-36-2048.jpg)





















