Embed presentation
Download to read offline

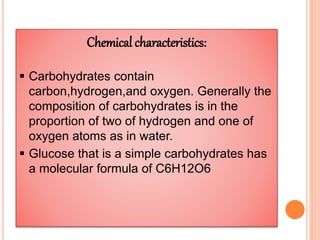

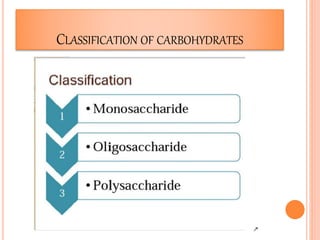

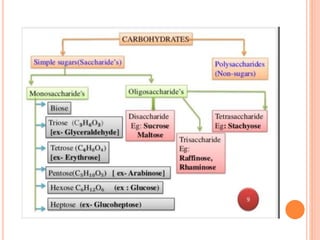

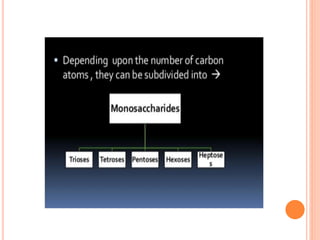
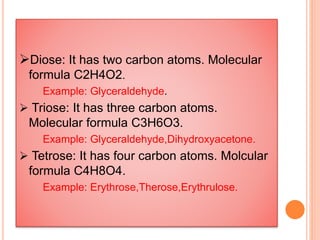


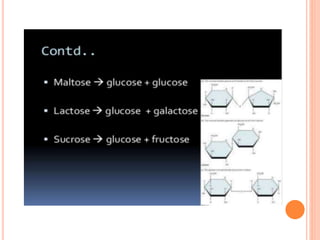

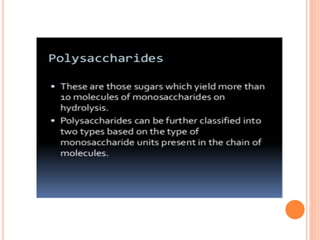
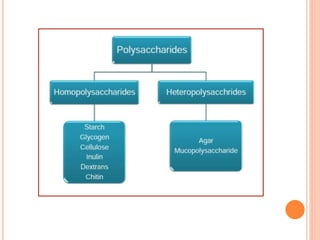

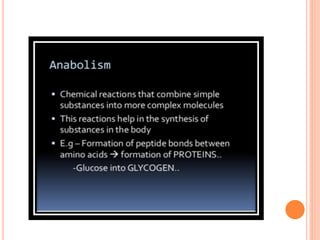
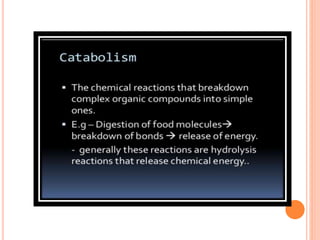

Carbohydrates are widely distributed in plants and animals. They function primarily as a fuel in the body by liberating energy through oxidation. Certain carbohydrate products also promote further oxidation of foods and can be used as starting materials for other biological compounds. Chemically, carbohydrates contain carbon, hydrogen, and oxygen in proportions similar to water, with glucose having the formula C6H12O6. Carbohydrates can be classified based on the number of carbon atoms in their molecular structure, ranging from di- to heptoses.




















