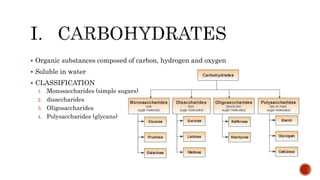
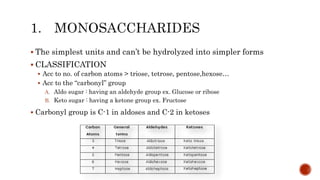
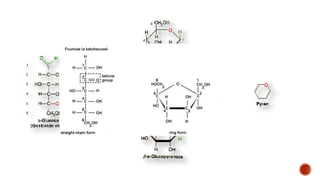
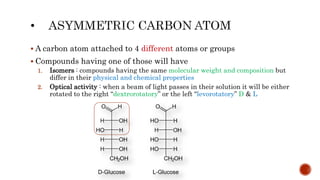
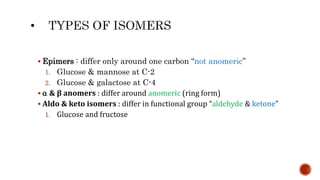
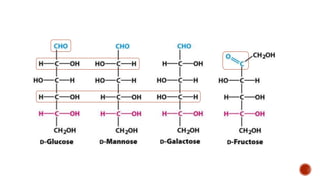
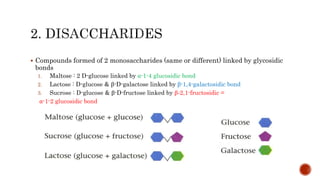
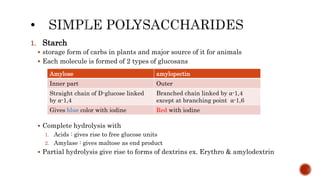
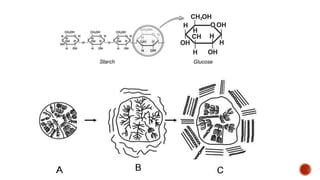
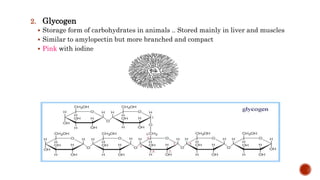
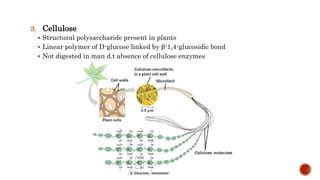
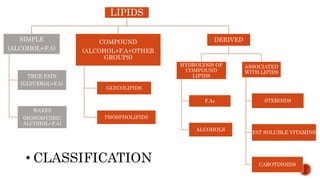
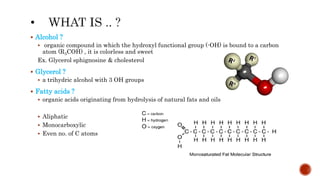
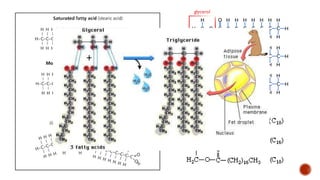
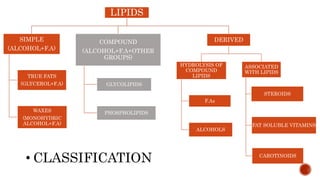
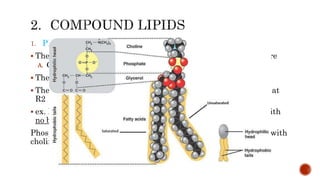
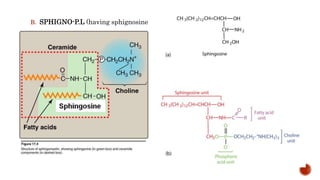
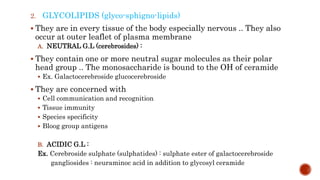
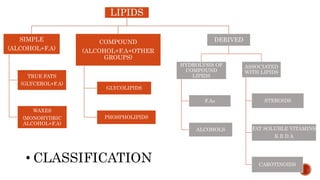
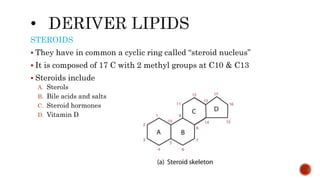
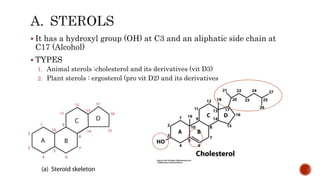
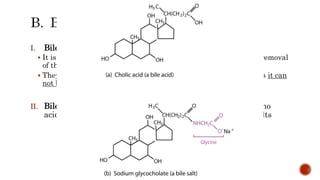
This document summarizes carbohydrates and lipids. It defines monosaccharides, disaccharides, and polysaccharides. It describes the classification, structures, and properties of common monosaccharides like glucose and fructose. It also discusses lipids, including fatty acids, glycerol, and classifications like fats, waxes, phospholipids, and glycolipids. Key biomolecules and roles are summarized such as phospholipids in cell membranes and glycolipids in nervous tissue.