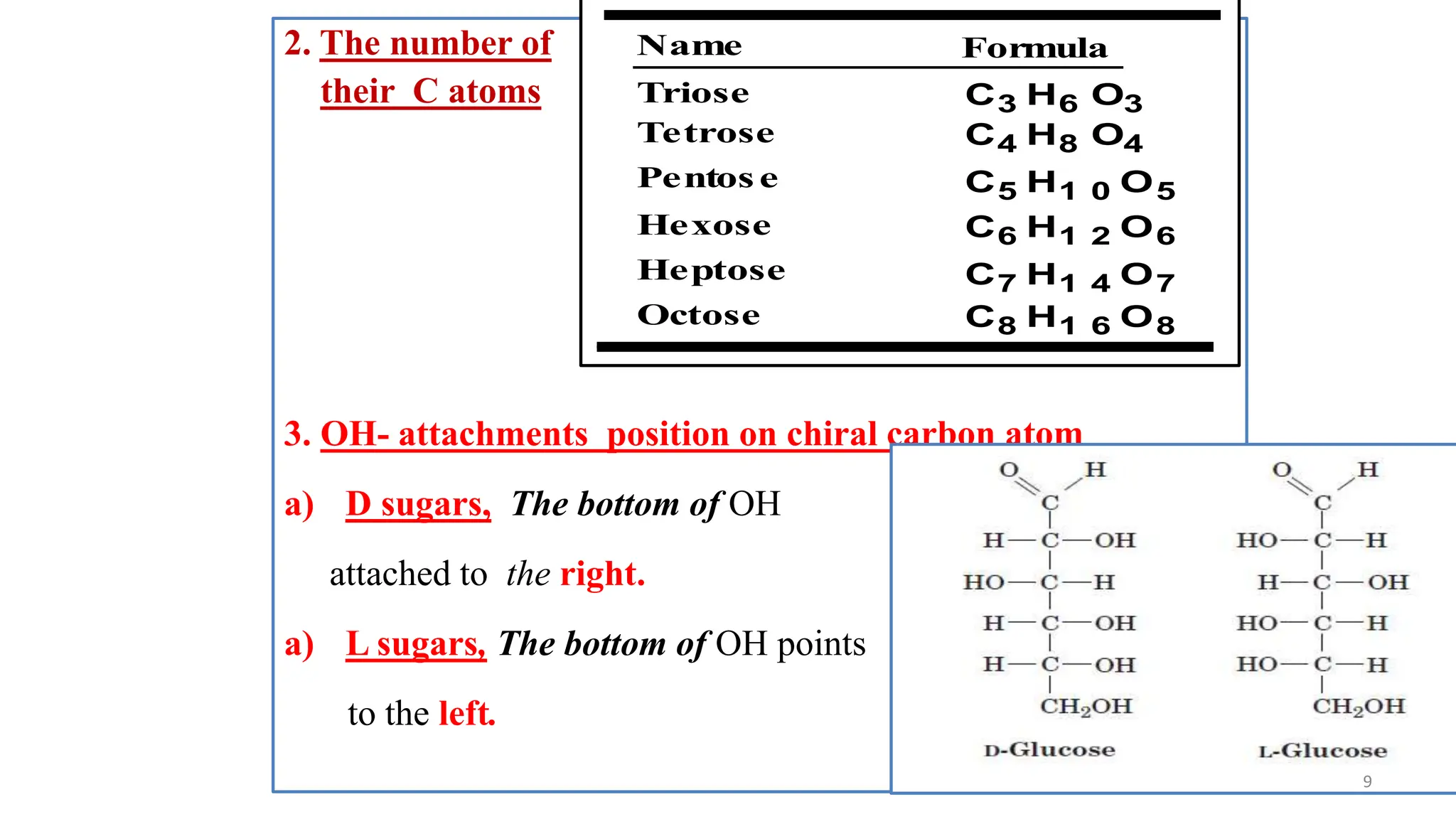
This document discusses biochemical molecules. It begins by explaining that all living organisms require biomolecules like organic and inorganic compounds. The four most common elements in living organisms are carbon, hydrogen, oxygen, and nitrogen. Biomolecules can be grouped as carbohydrates, lipids, proteins, nucleic acids, water, and minerals. Carbohydrates include monosaccharides, disaccharides, and polysaccharides. Lipids function as storage, structure, signaling, and pigments. Proteins are made of amino acids linked by peptide bonds that can fold into complex structures.