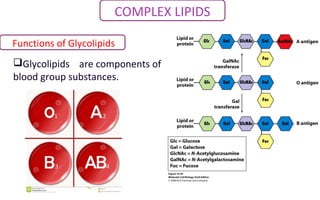
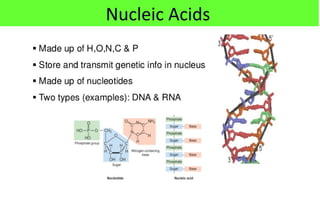
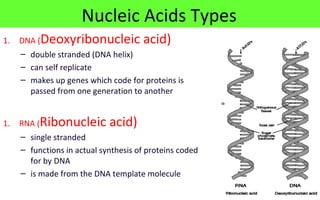
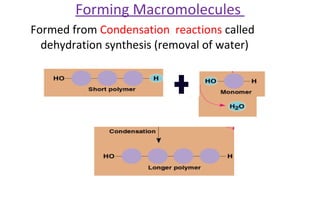
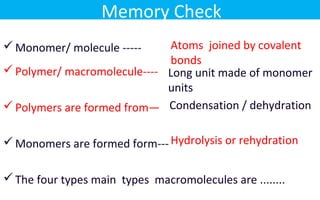
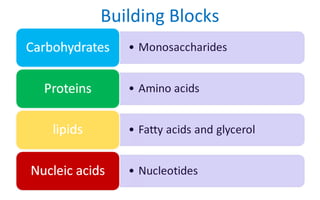
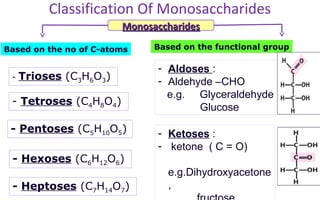
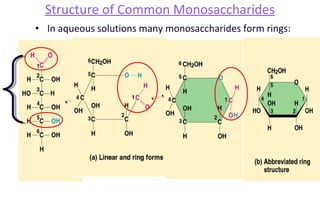
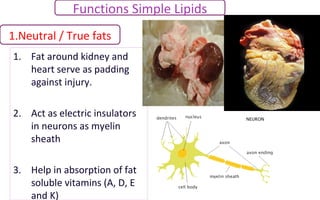
This document discusses various types of macromolecules including carbohydrates, lipids, proteins, and nucleic acids. It begins by defining biochemistry and explaining that it studies the chemical reactions that occur in living organisms, focusing on substances like enzymes, hormones, carbohydrates, proteins, lipids, DNA and RNA. It then discusses the importance of biochemistry in pharmacy and nursing, explaining how it helps understand drug constitution, metabolism, storage and biochemical tests. The document proceeds to discuss carbohydrates in depth, explaining their classification into mono-, di-, oligo- and polysaccharides. It provides examples and functions of important carbohydrates like glucose, fructose, starch and cellulose. Finally, it briefly introduces lipids and















































![Uses Waxes
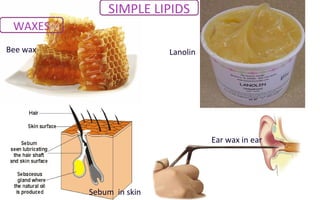
SIMPLE LIPIDS
Lanolin is frequently used in
protective baby skin treatment and
as a treatment for sore nipples in
breastfeeding mothers.[13]
Shellac wax is used in dental
technology, in (partial) denture
production.](https://image.slidesharecdn.com/macromolecules-150911133121-lva1-app6892/85/Macromolecules-57-320.jpg)