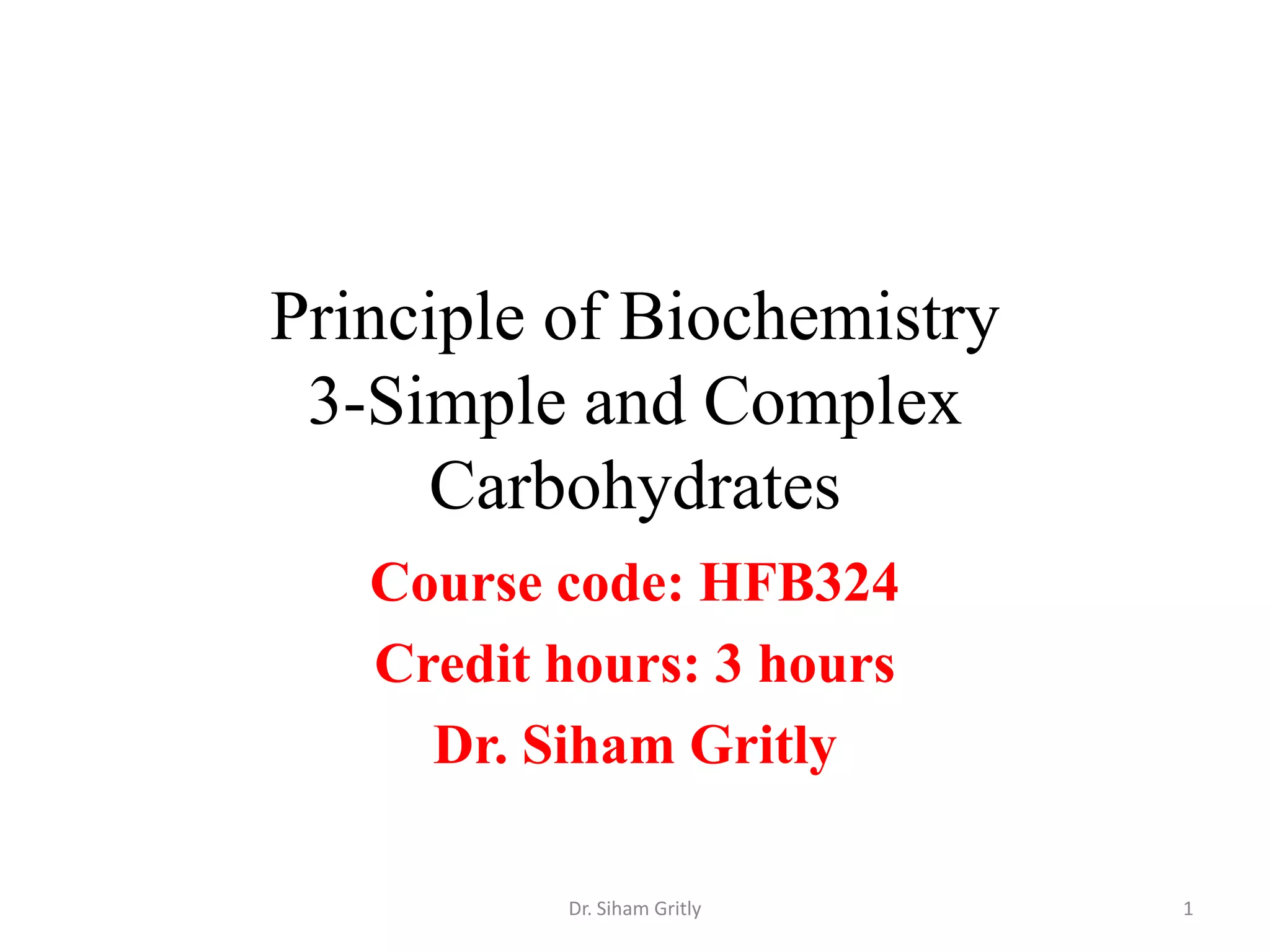
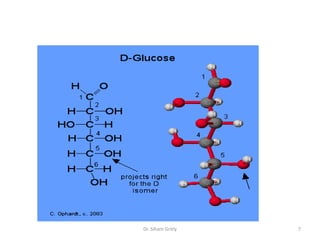
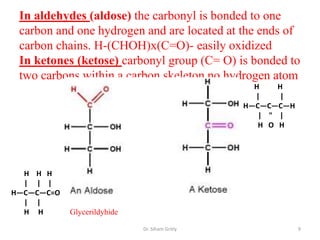
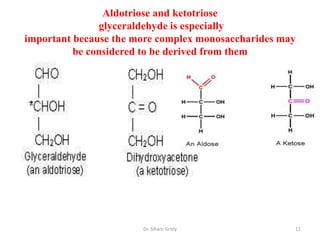
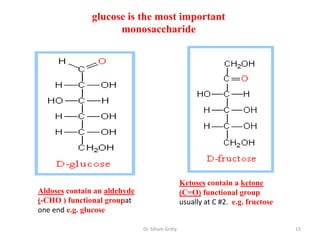
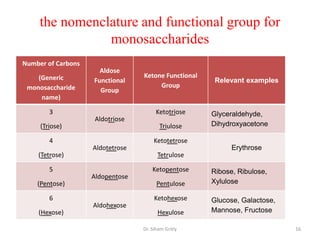
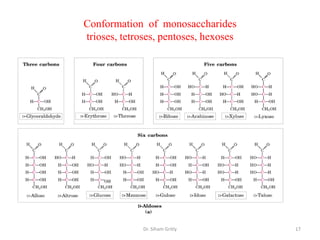
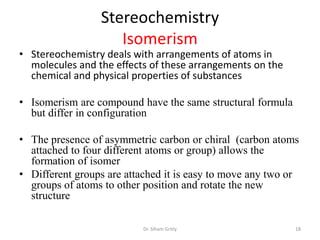
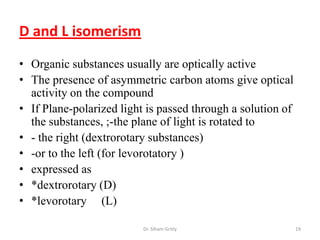
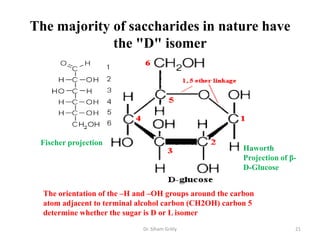
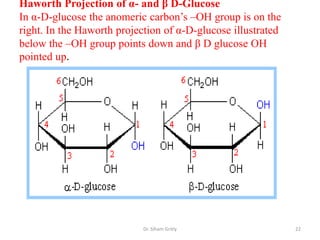
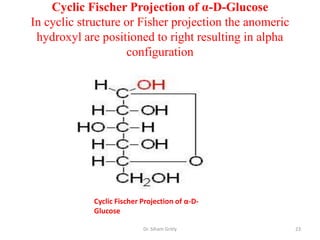
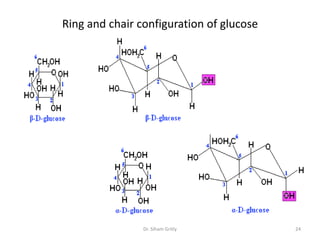
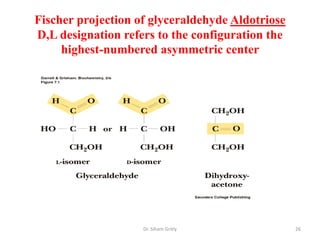
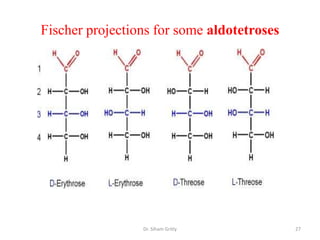
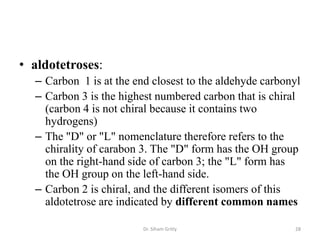
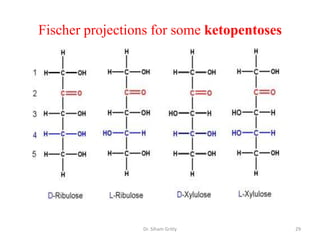
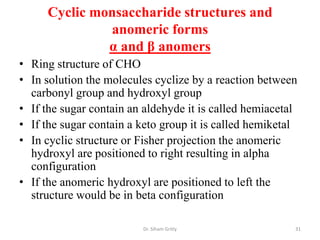
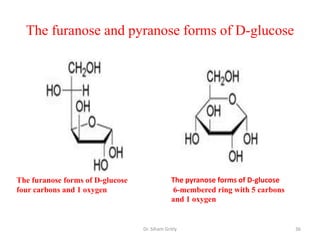
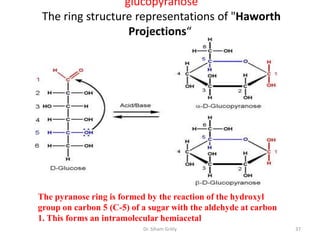
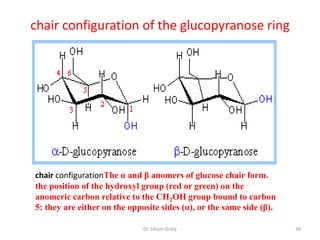
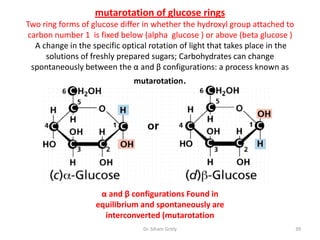
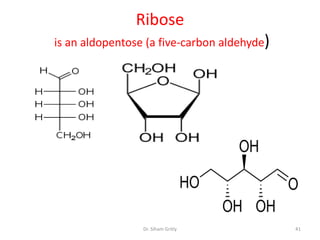
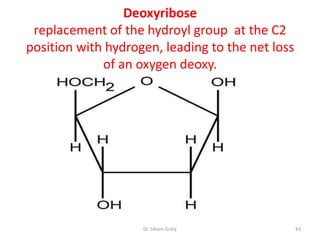
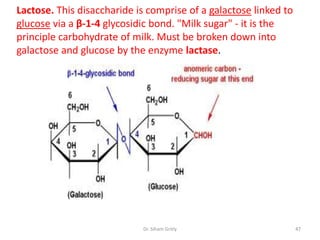
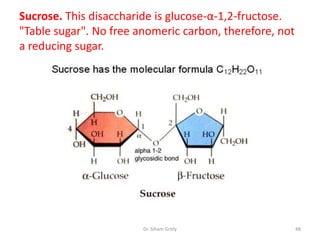
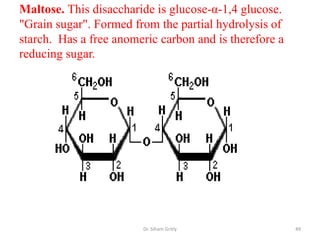
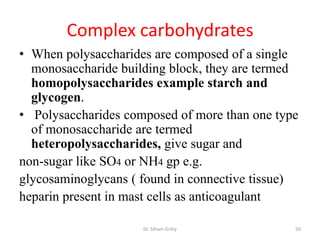
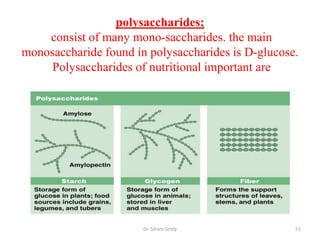
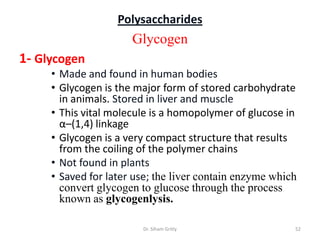
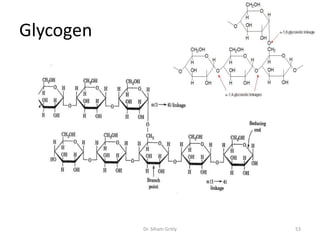
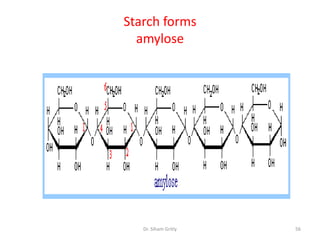
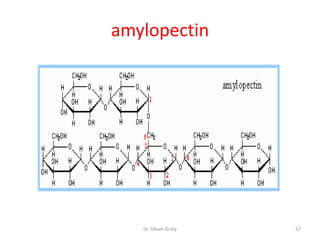
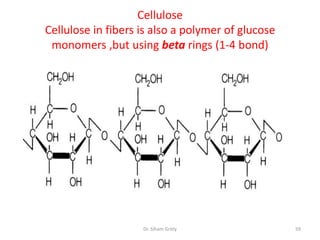
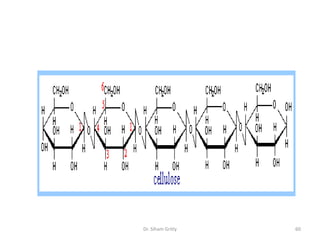
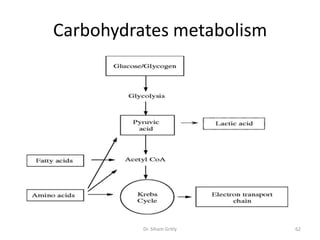
The document discusses carbohydrate nomenclature and structures. It defines carbohydrates as polyhydroxy aldehydes or ketones made of carbon, oxygen, and hydrogen. Monosaccharides include aldoses with an aldehyde group and ketoses with a ketone group. Common monosaccharides are glucose, galactose, and fructose which are hexoses. The document discusses cyclic forms such as pyranoses and furanoses. It also covers stereochemistry, anomers, chair conformations, and mutarotation.