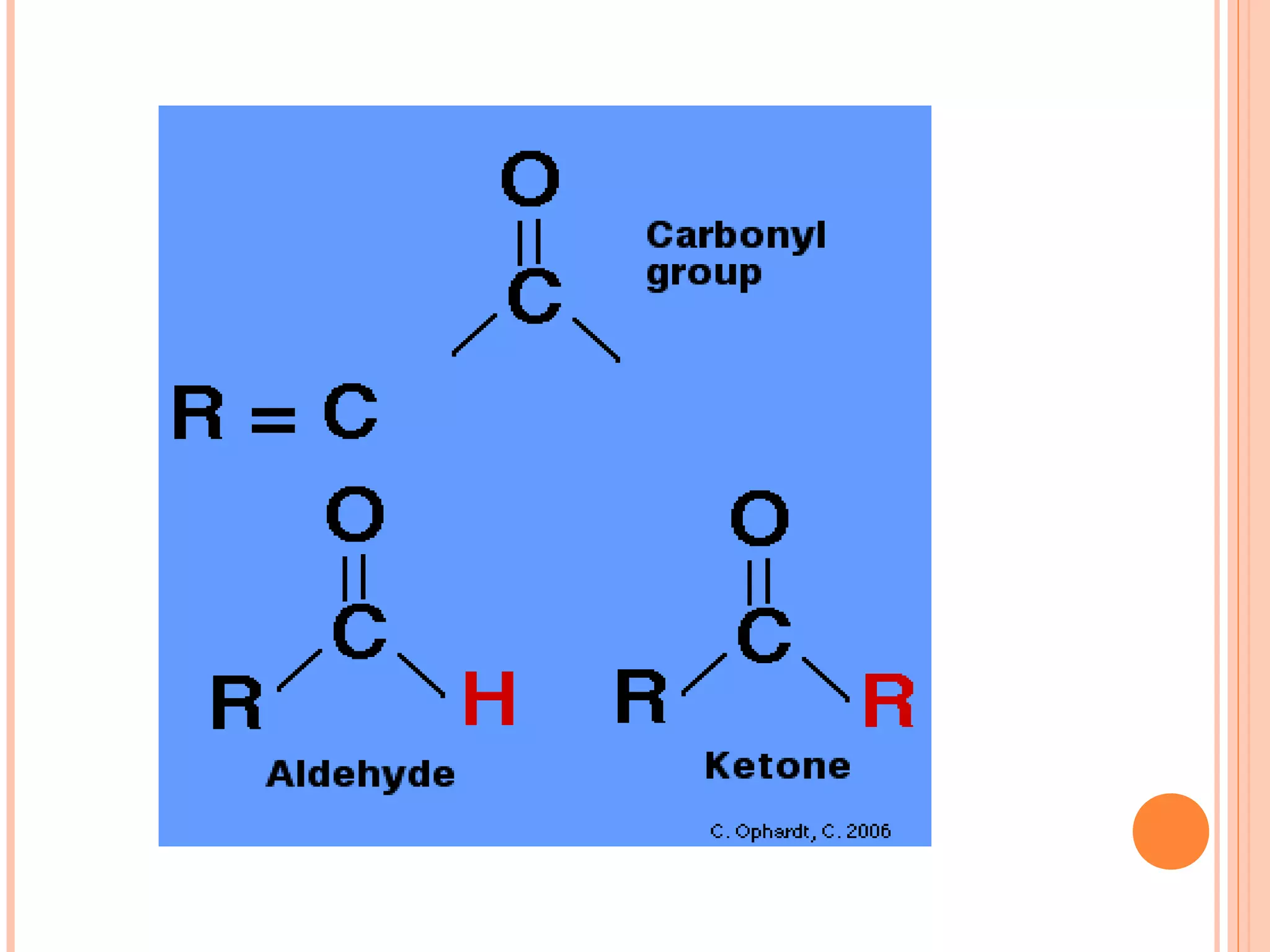
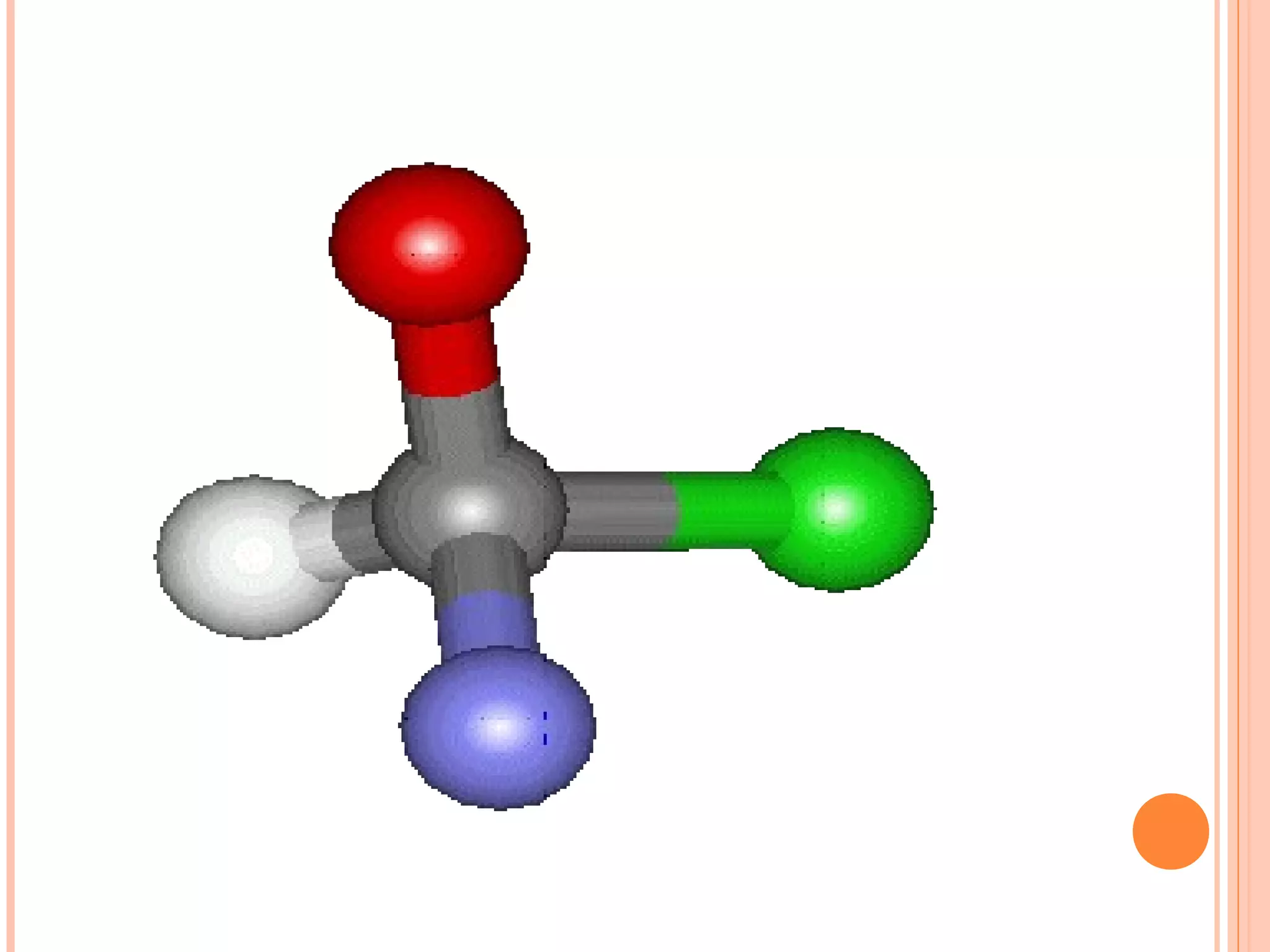
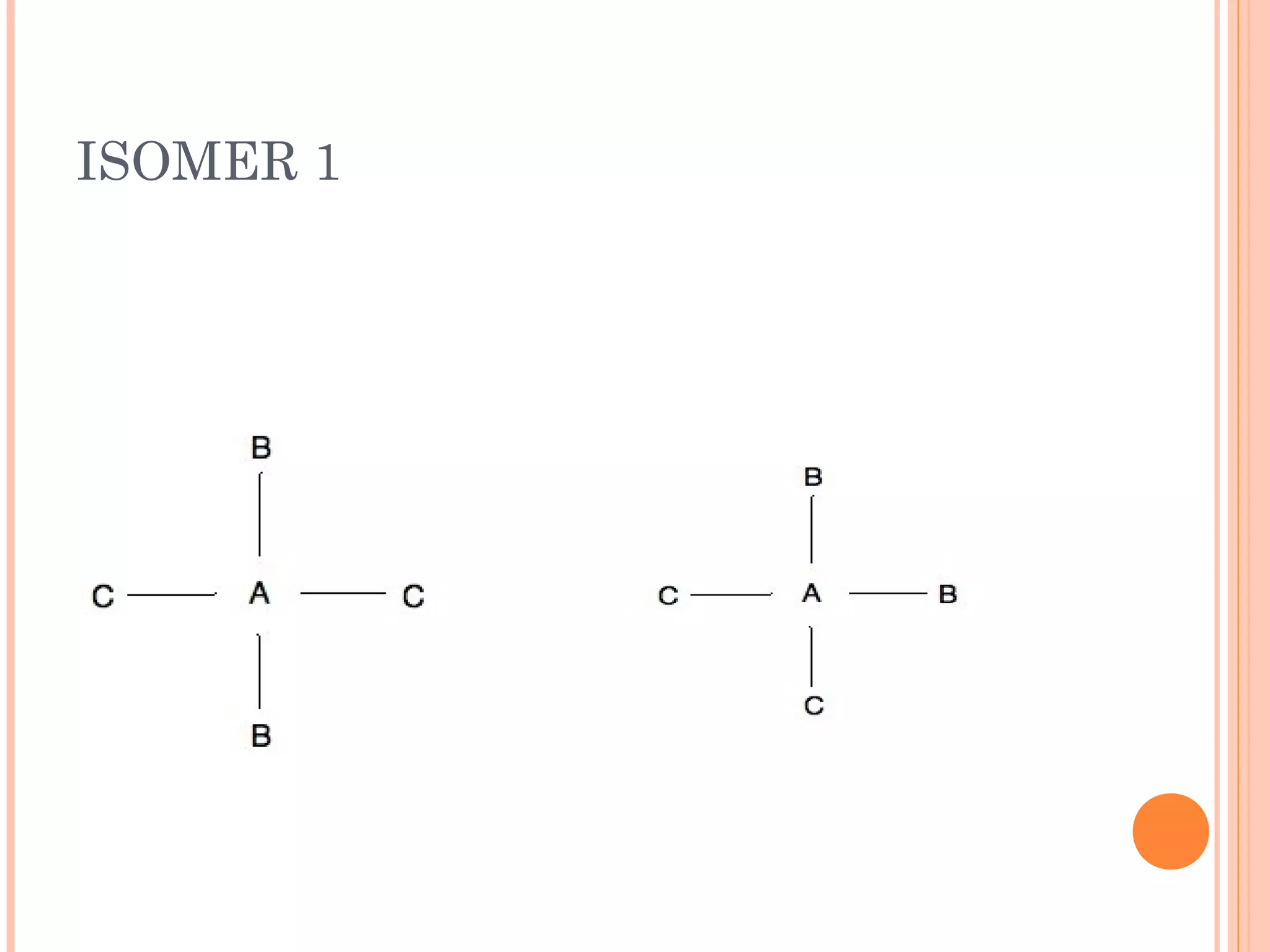
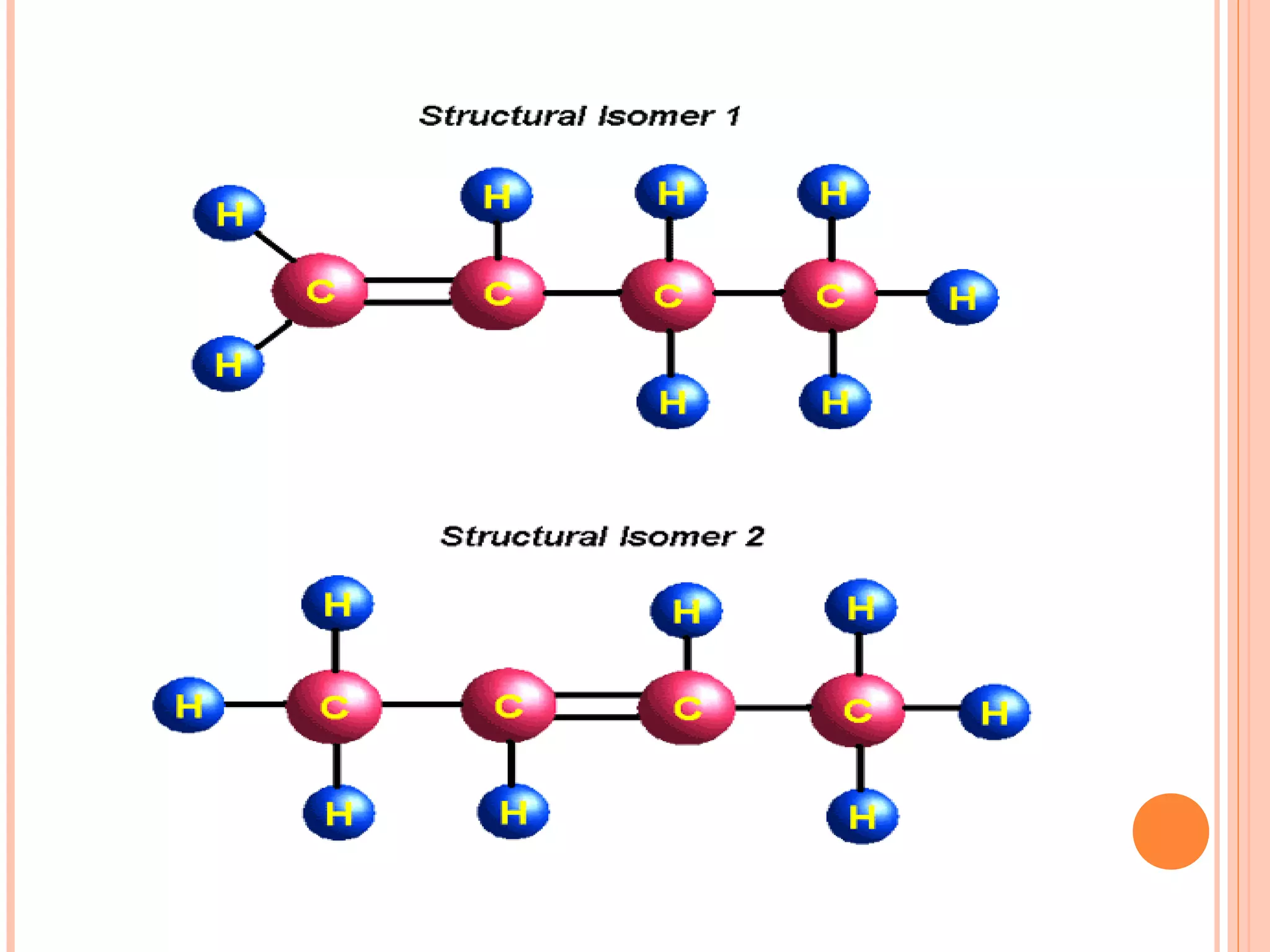
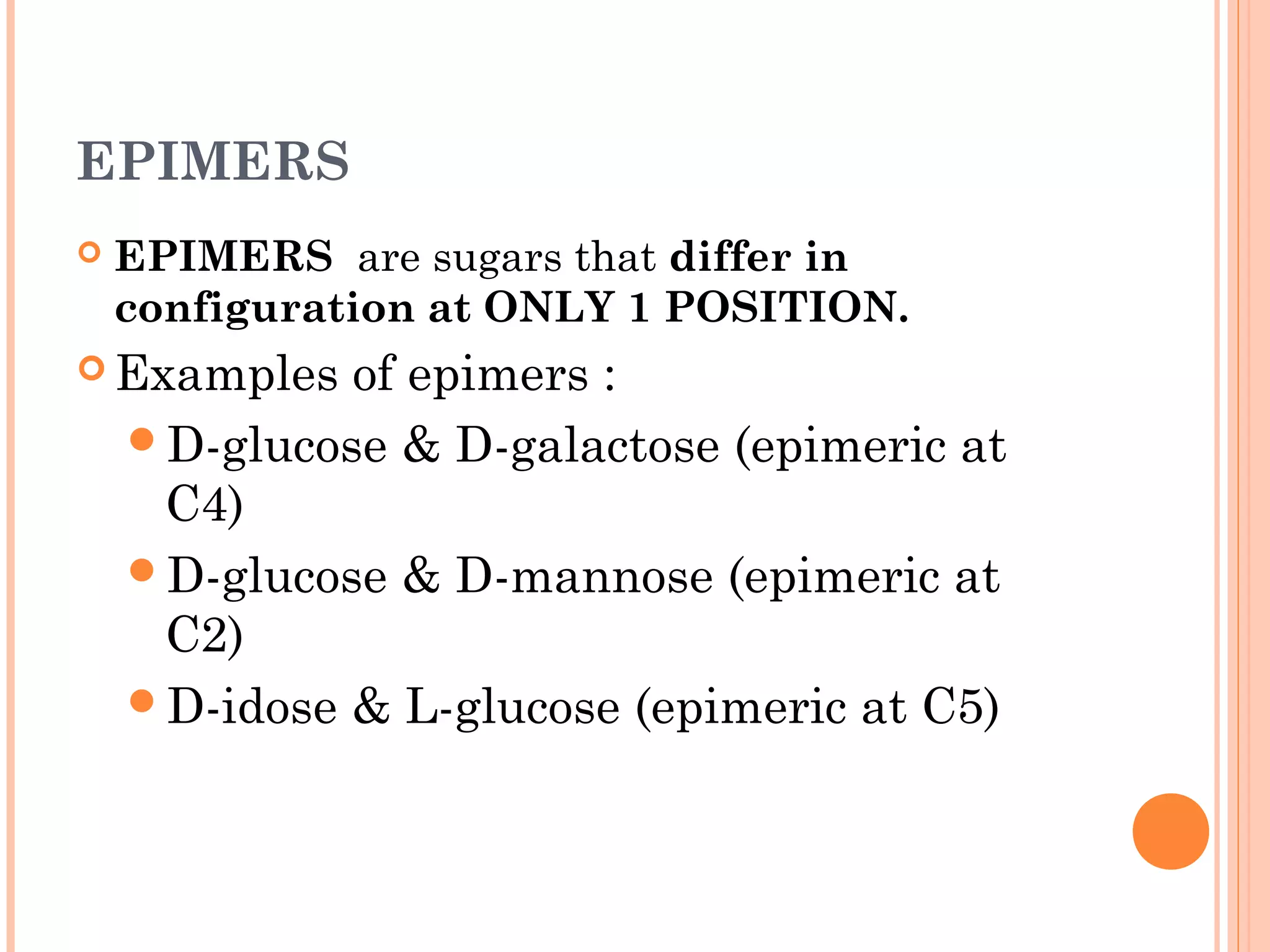
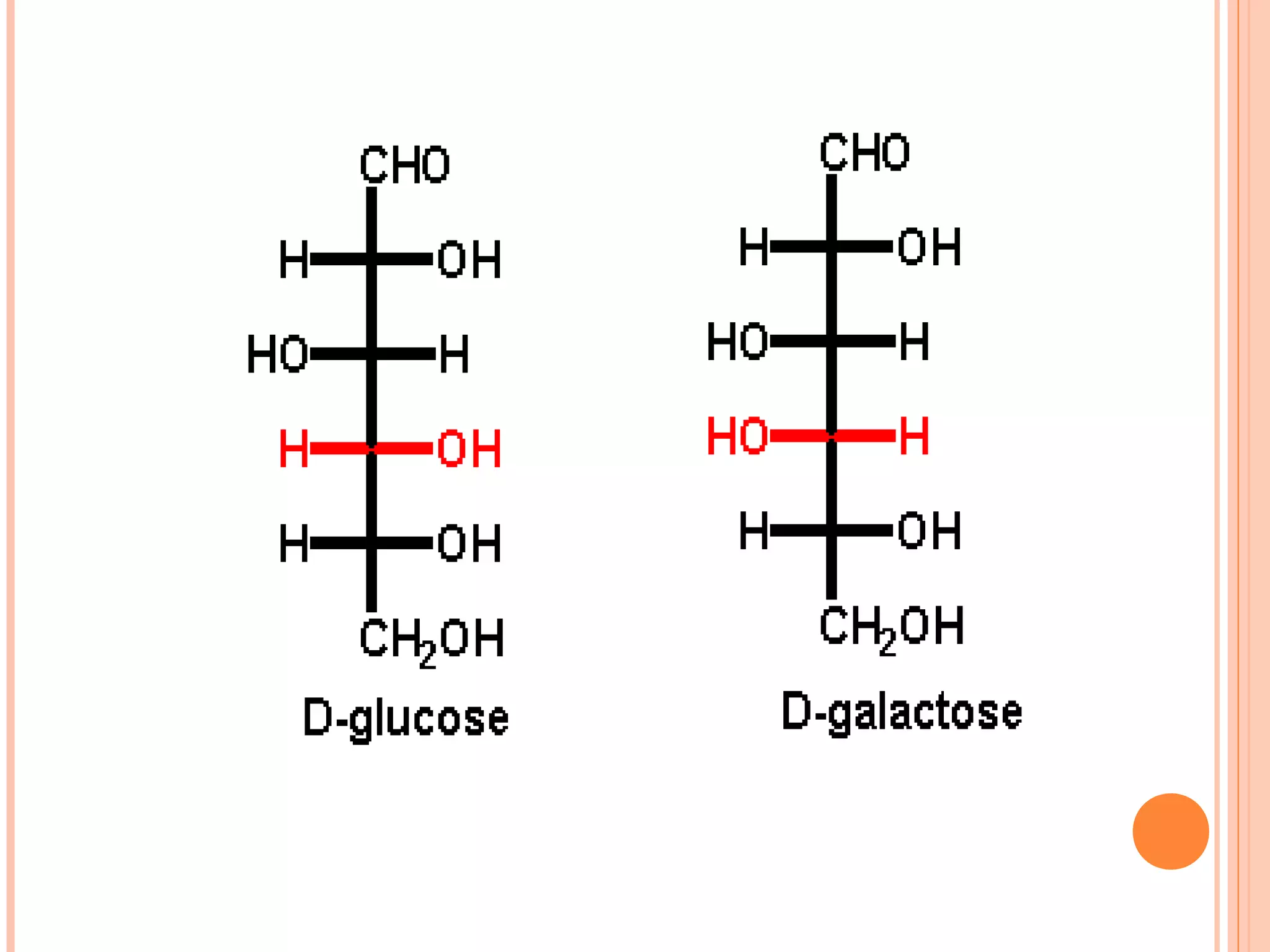
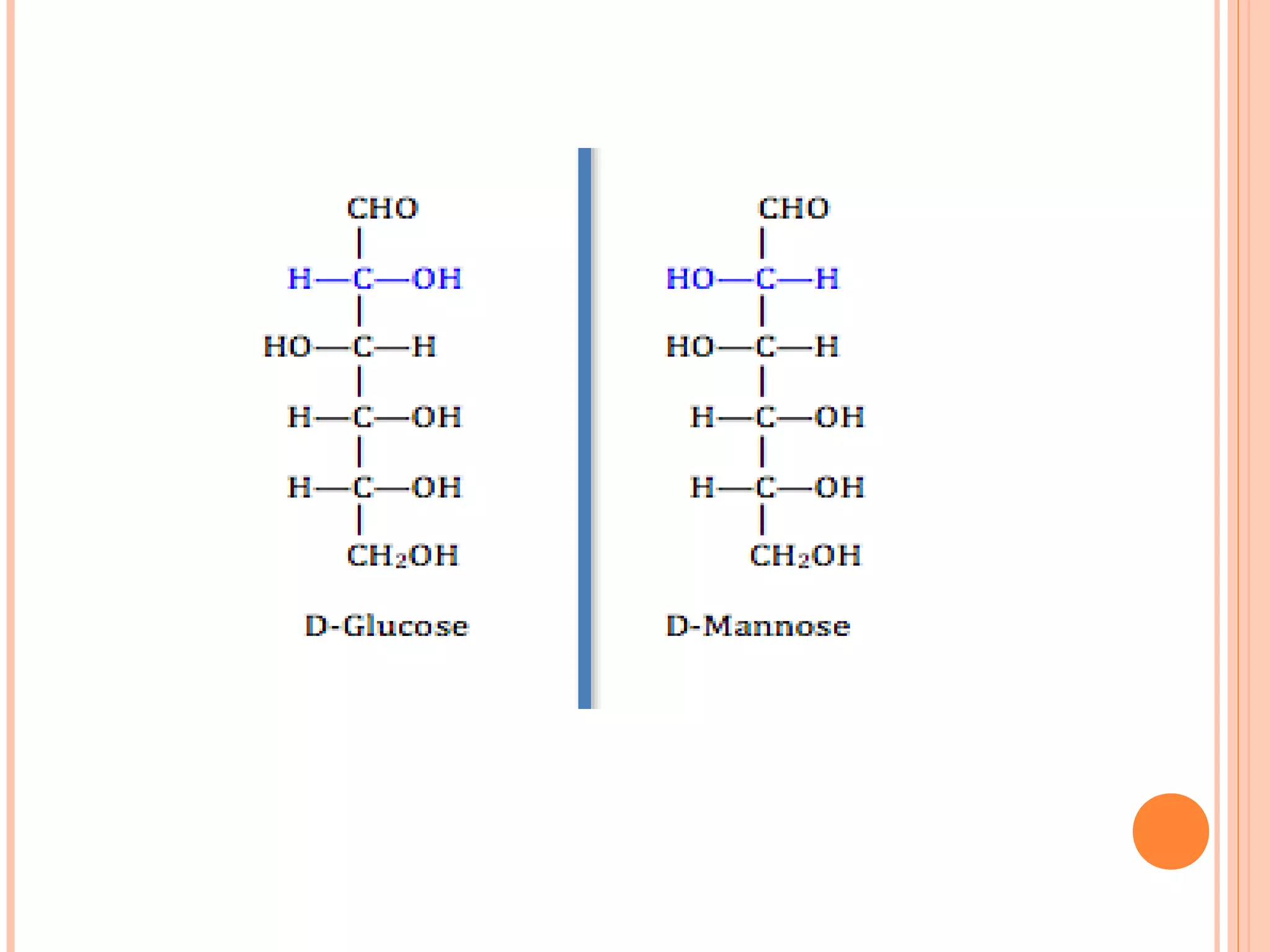
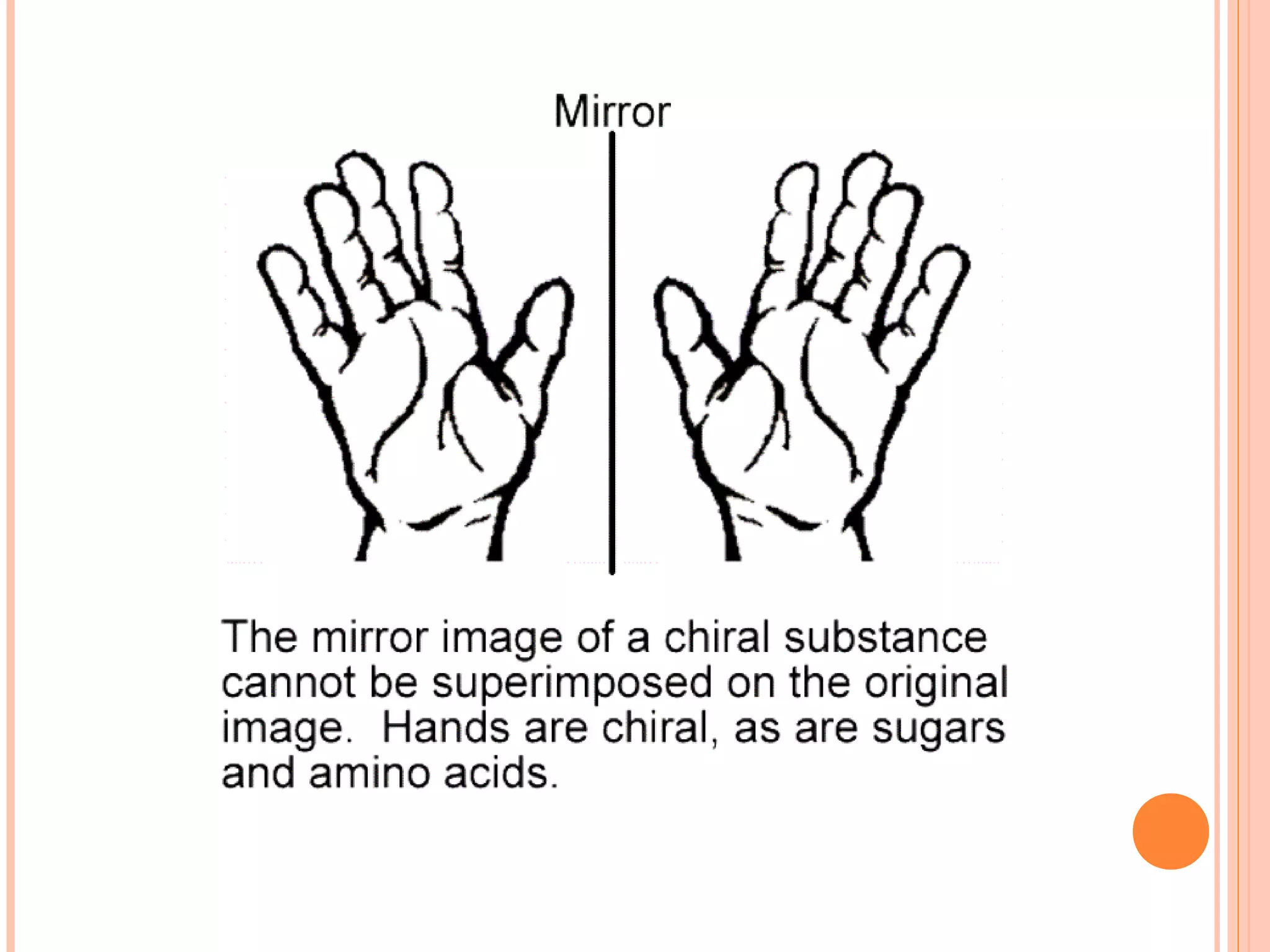
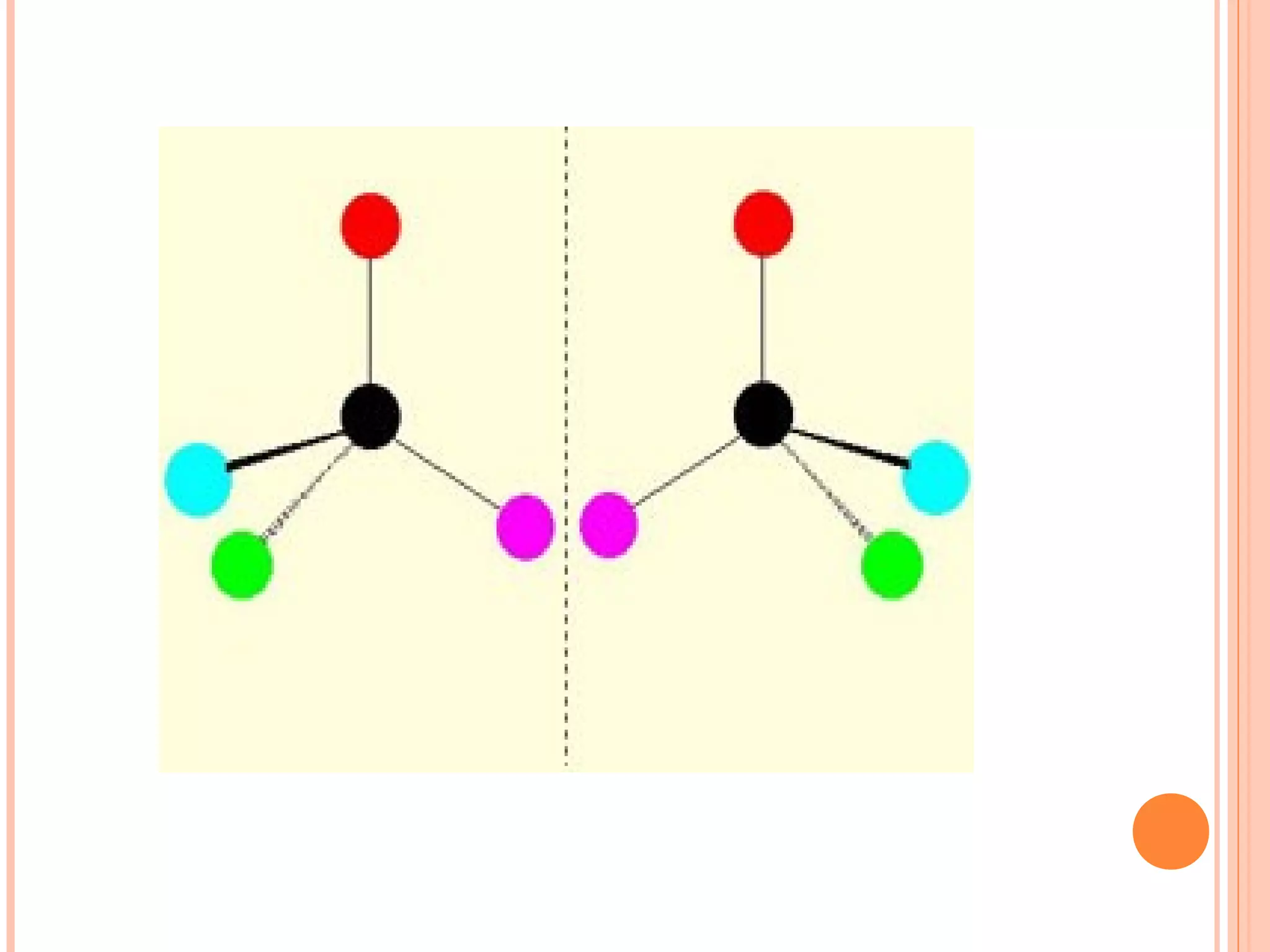
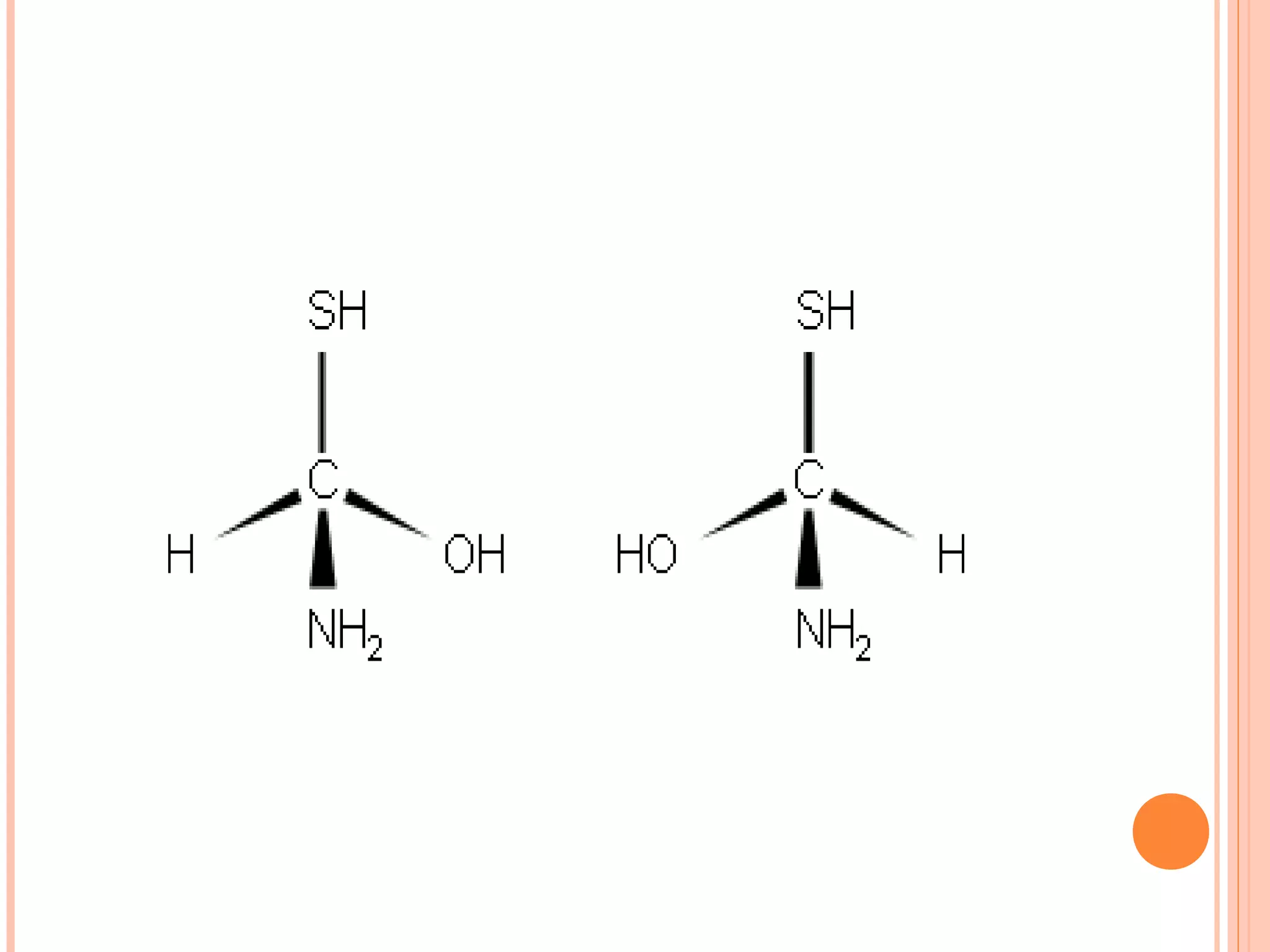
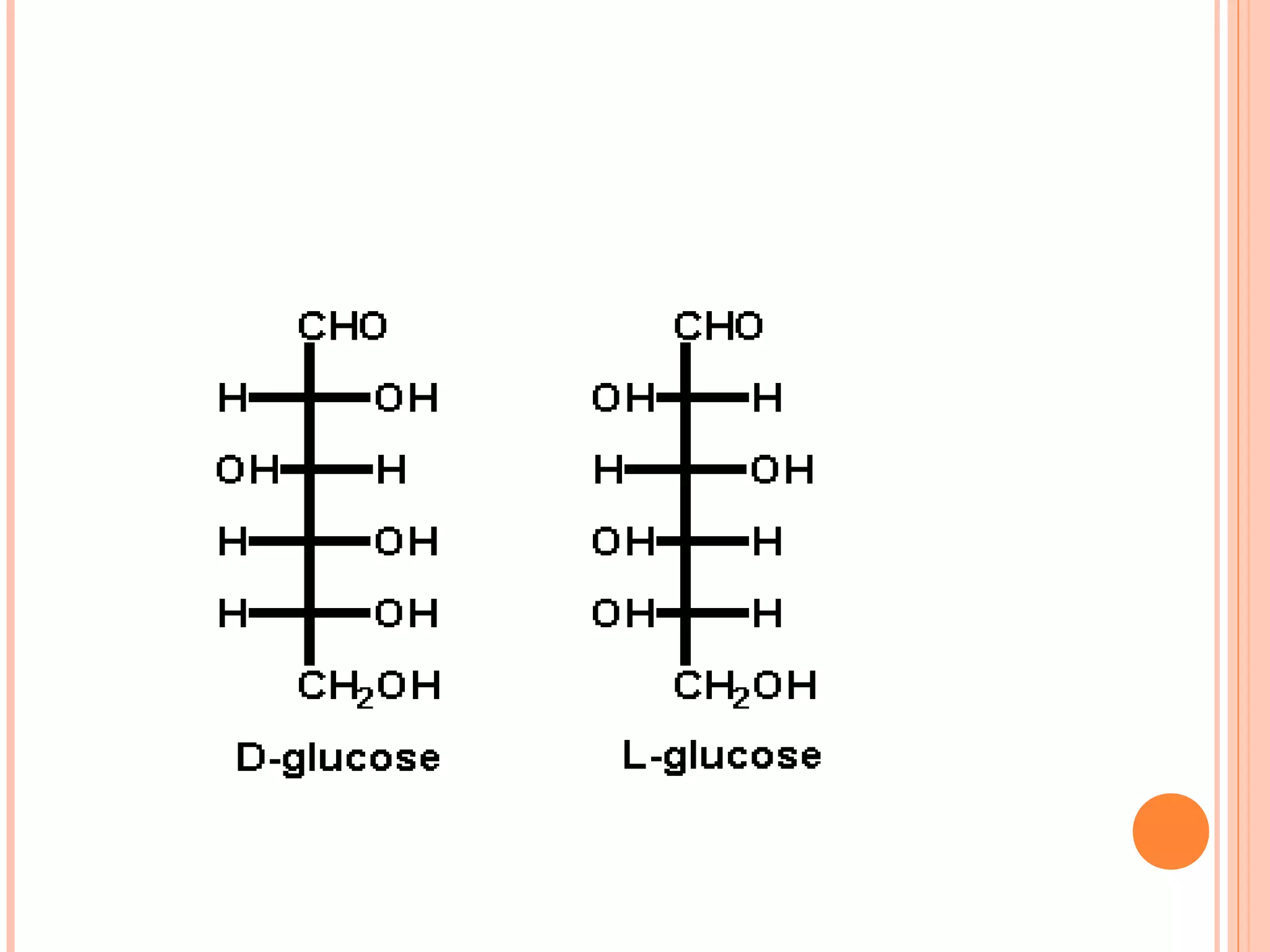
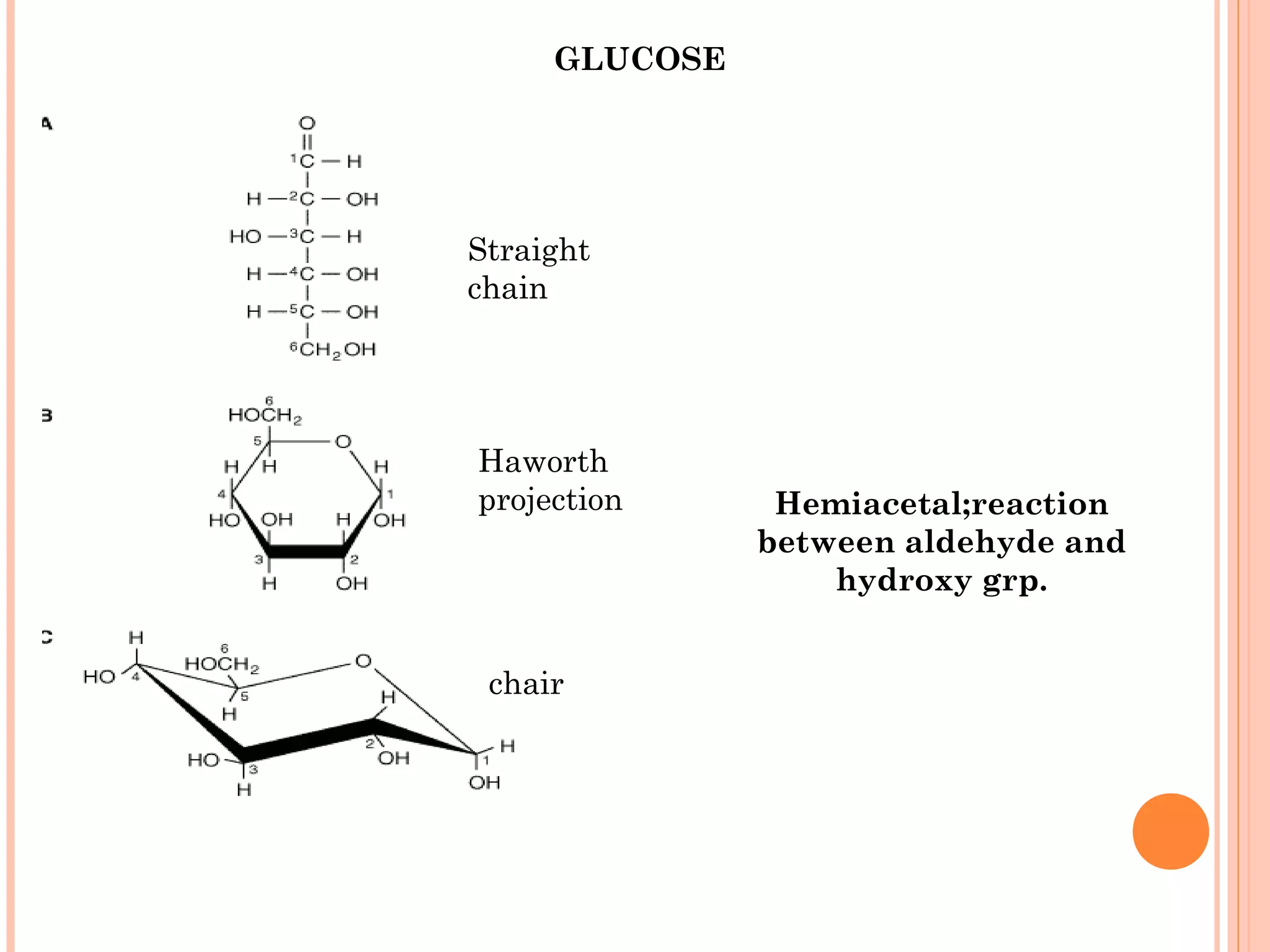
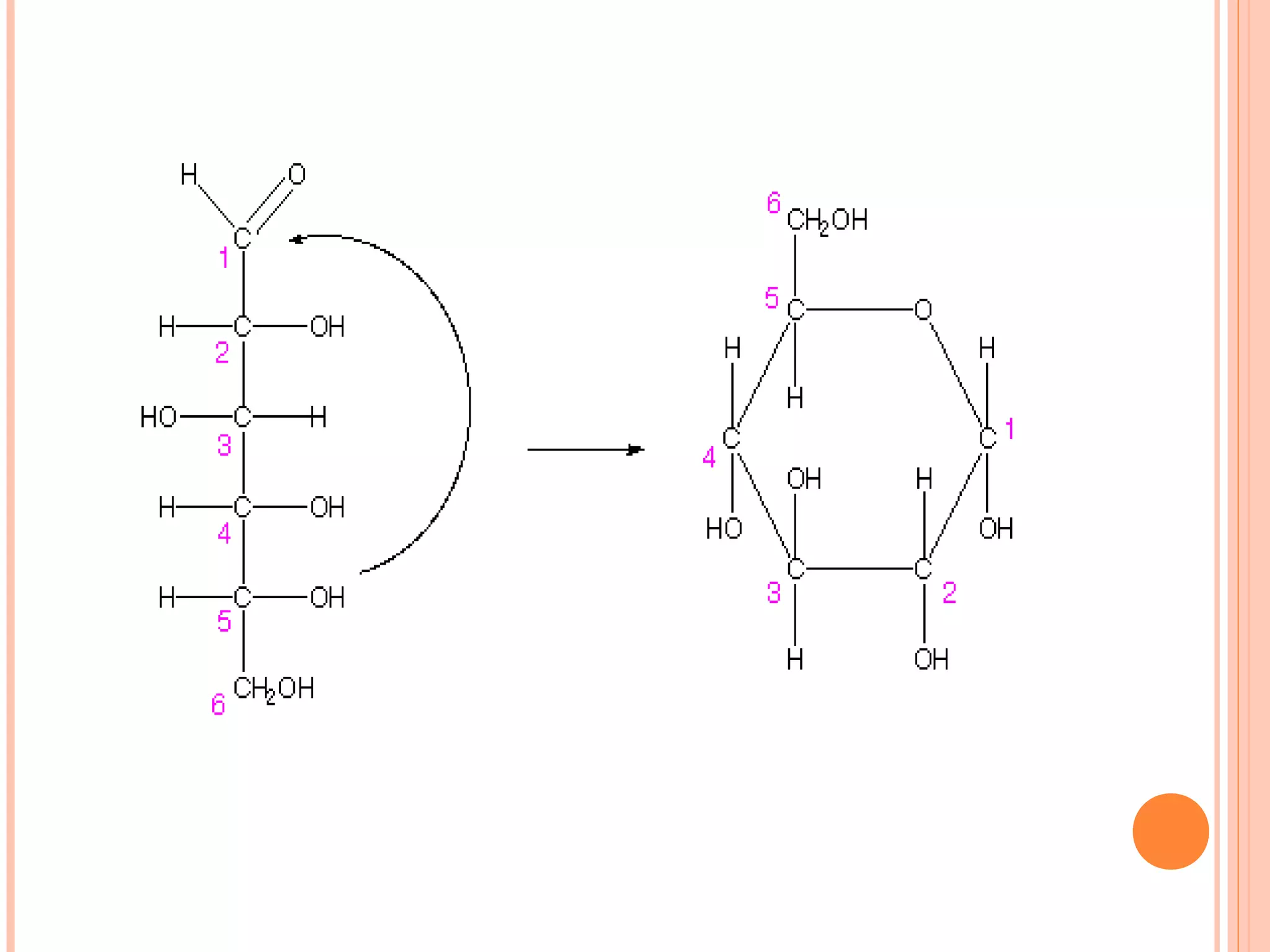
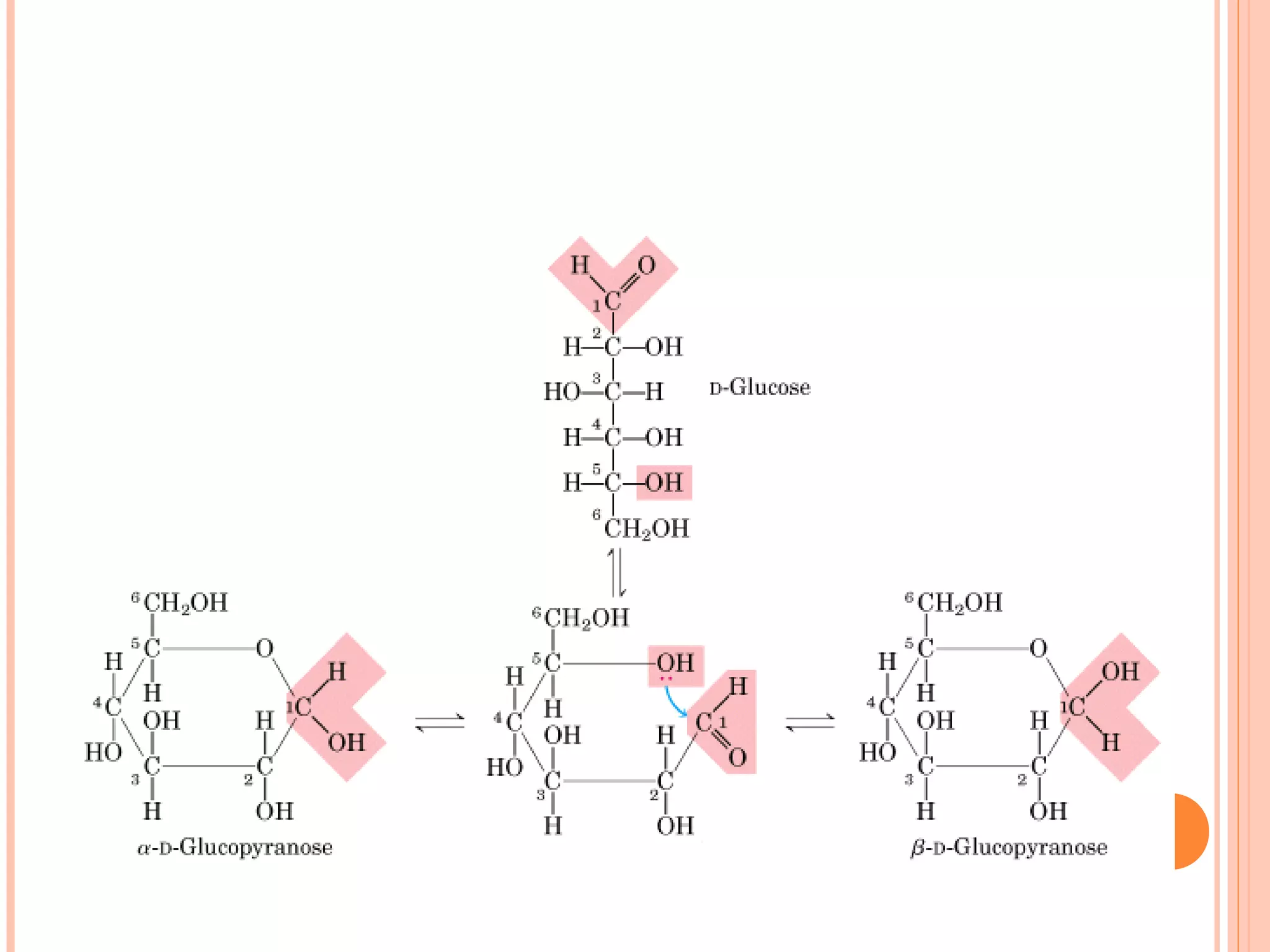
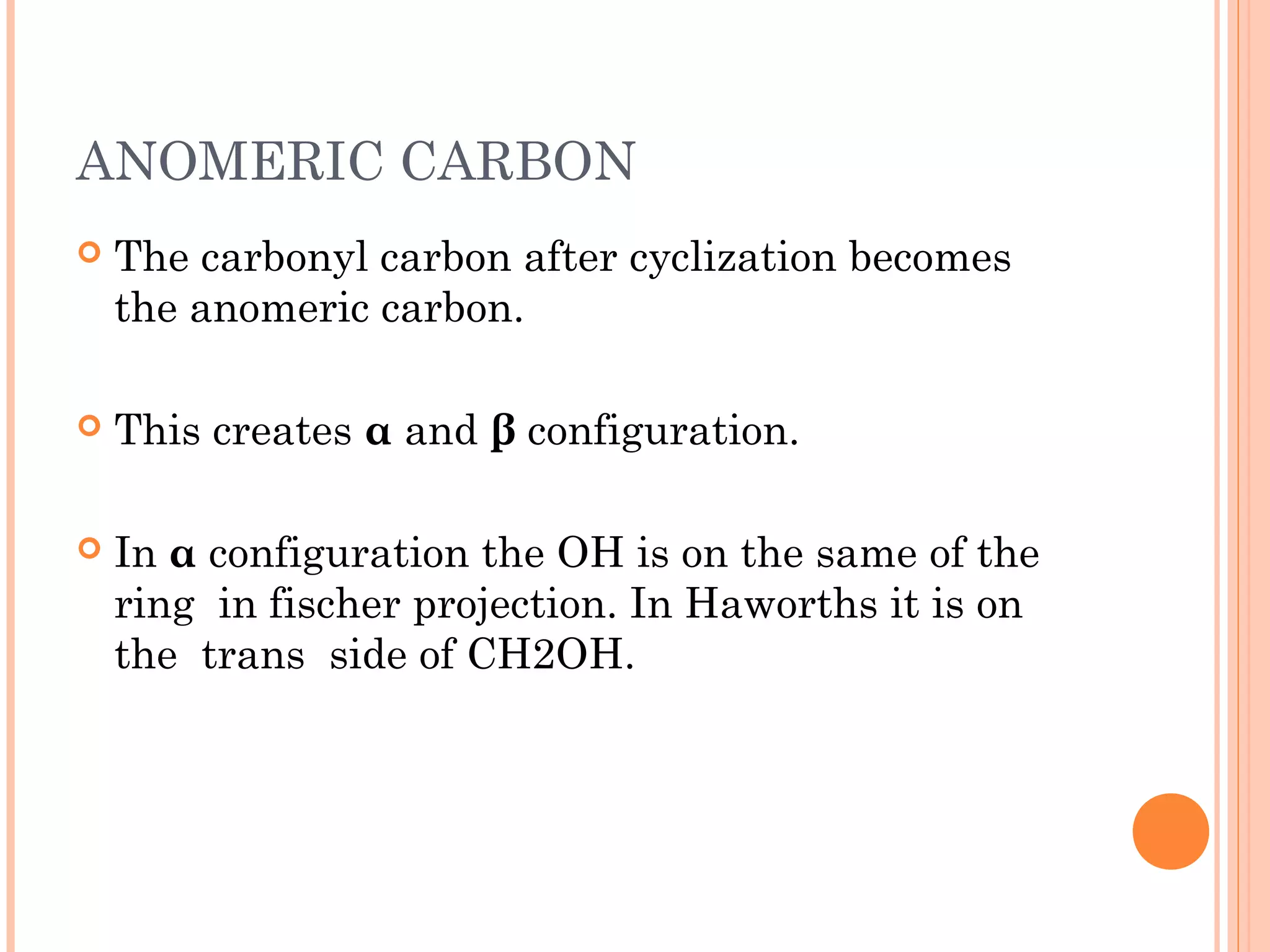
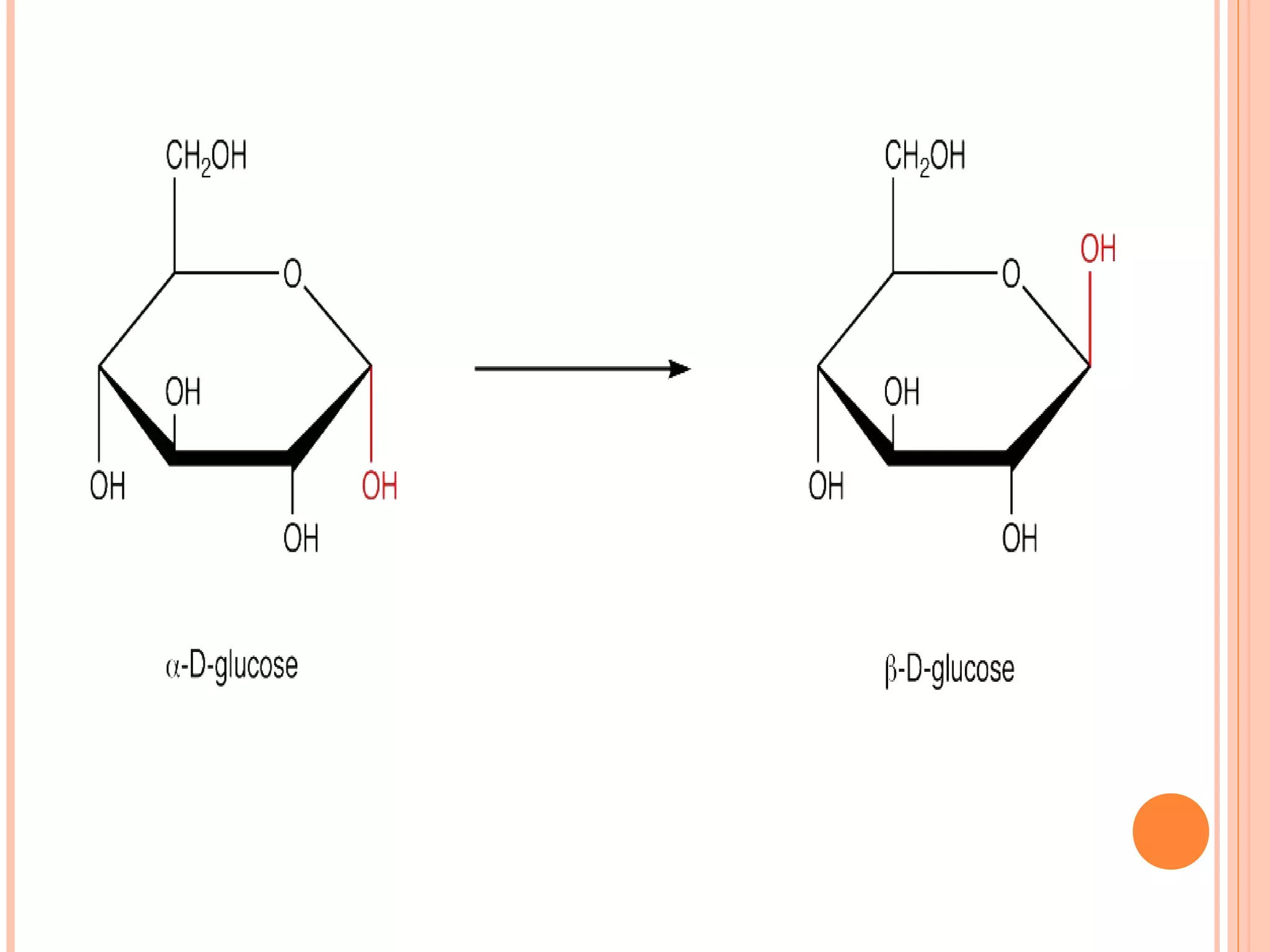
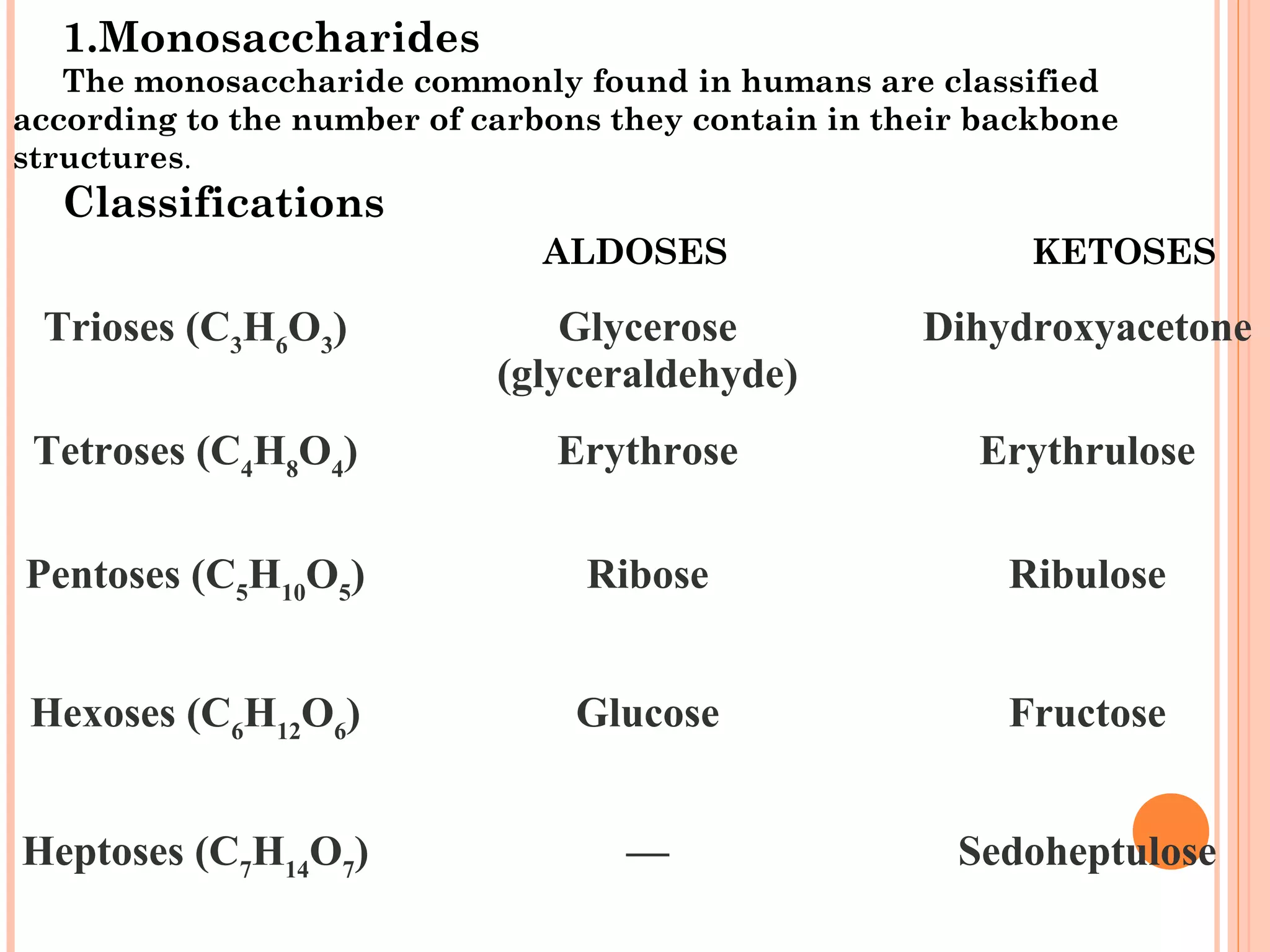
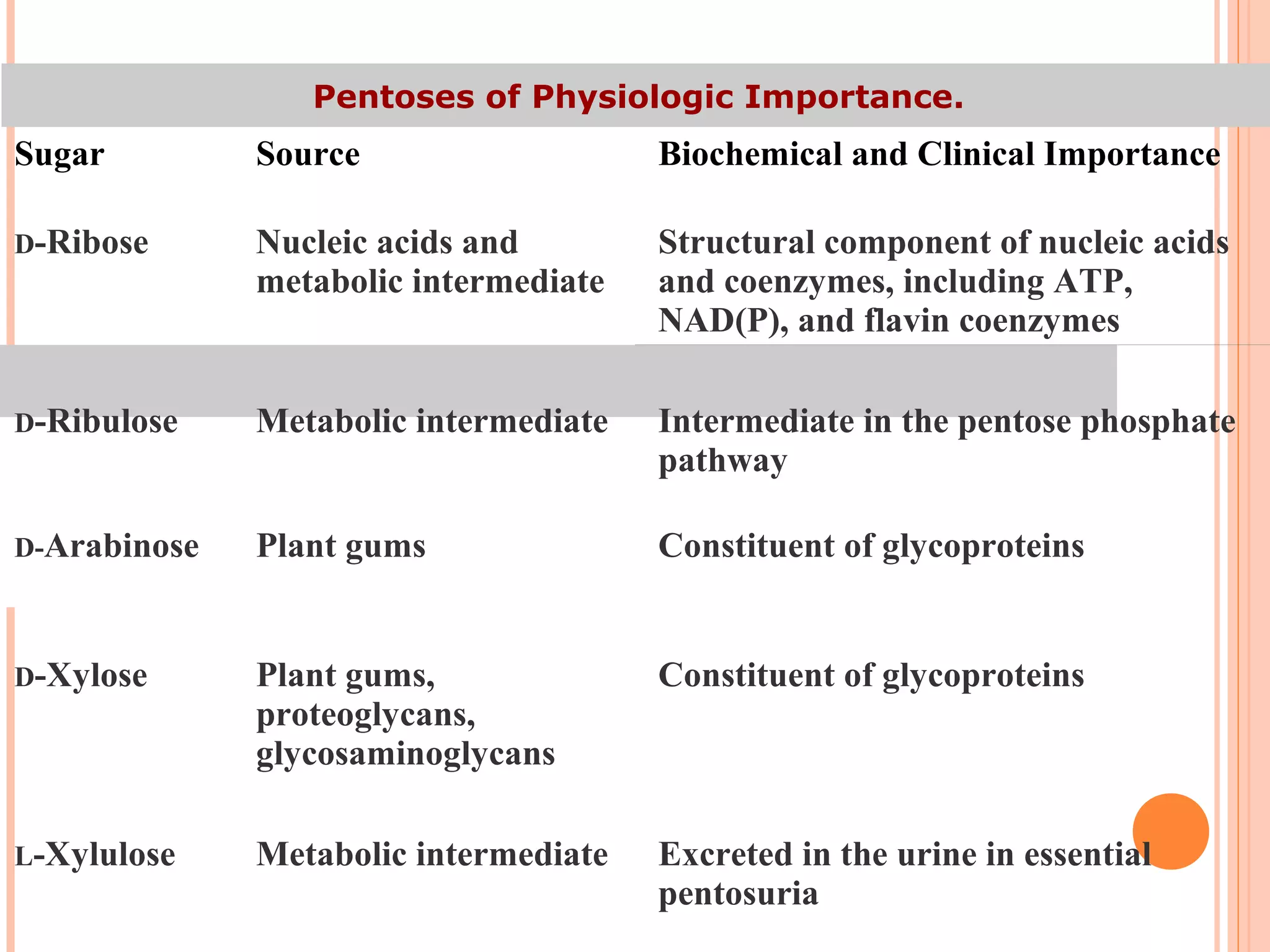
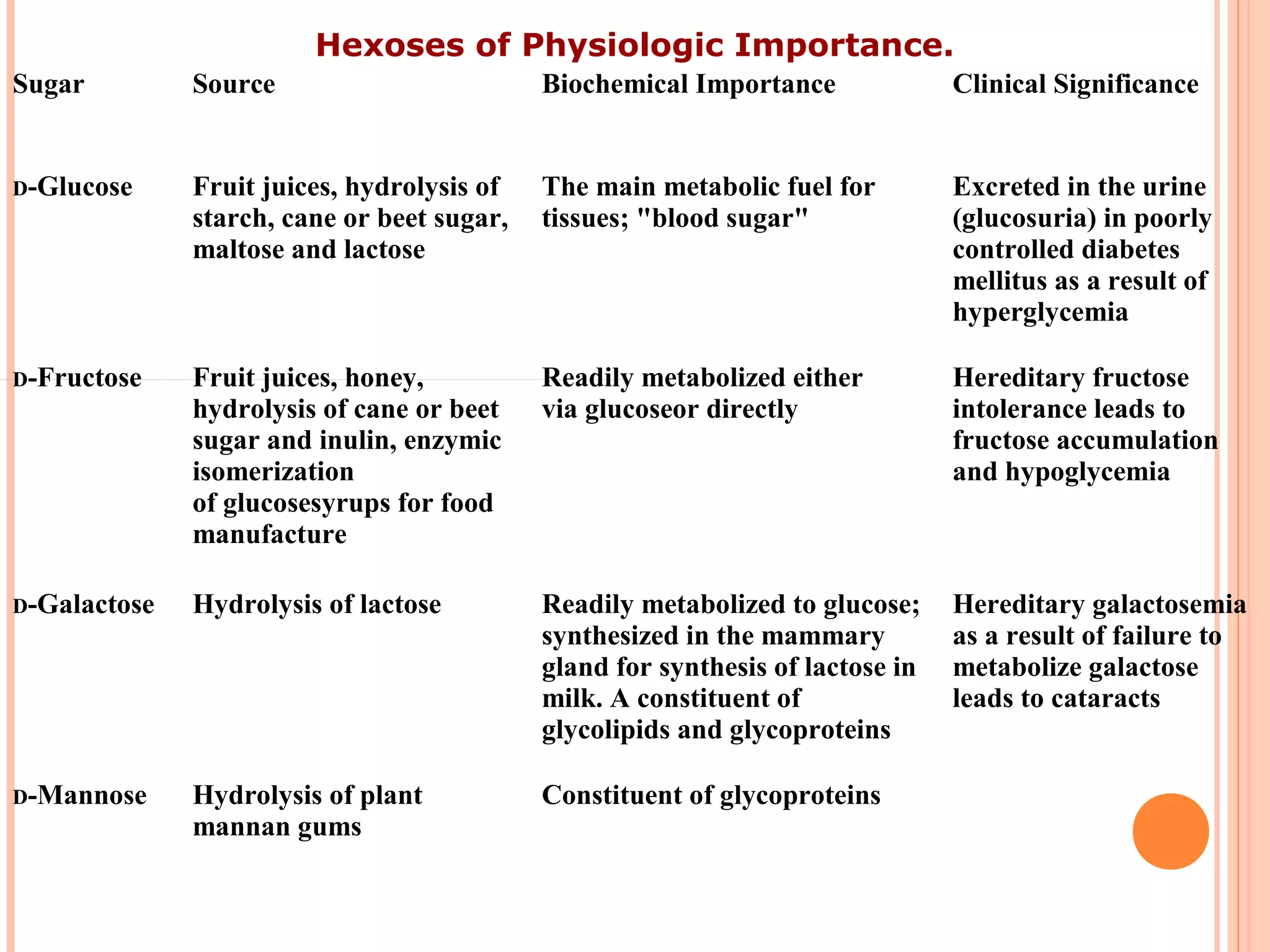
Carbohydrates are one of the major classes of biological molecules and are the most abundant. They have many important functions including energy storage, structural components, and cell signaling. Carbohydrates exist as monomers, dimers, oligomers, and polymers. Monosaccharides like glucose and fructose are the simplest units and exist as open chains or rings. Isomers have the same molecular formula but different structures. Cyclization of monosaccharides forms rings called pyranoses and furanoses. The document defines important carbohydrate terms and classifications.