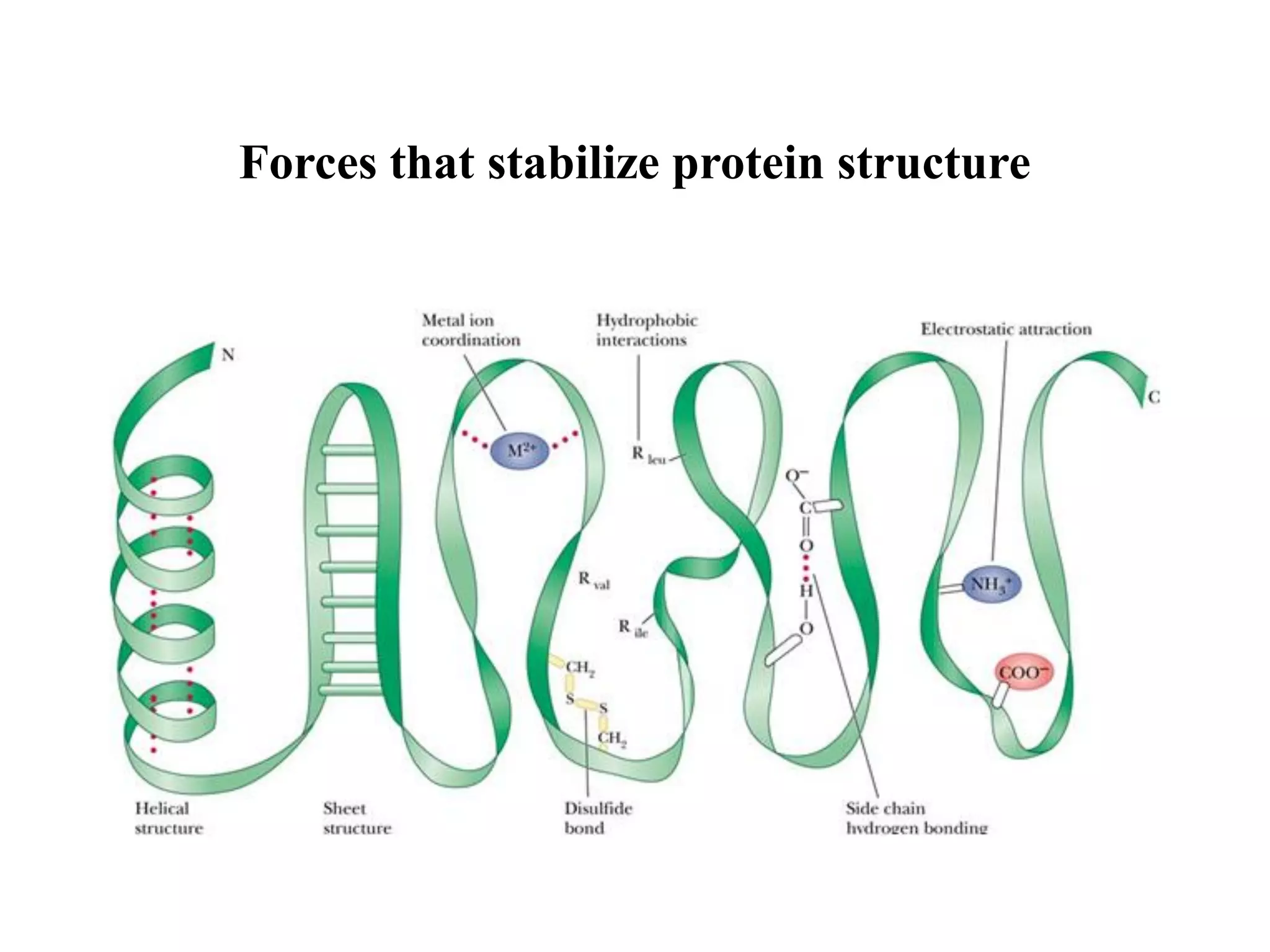
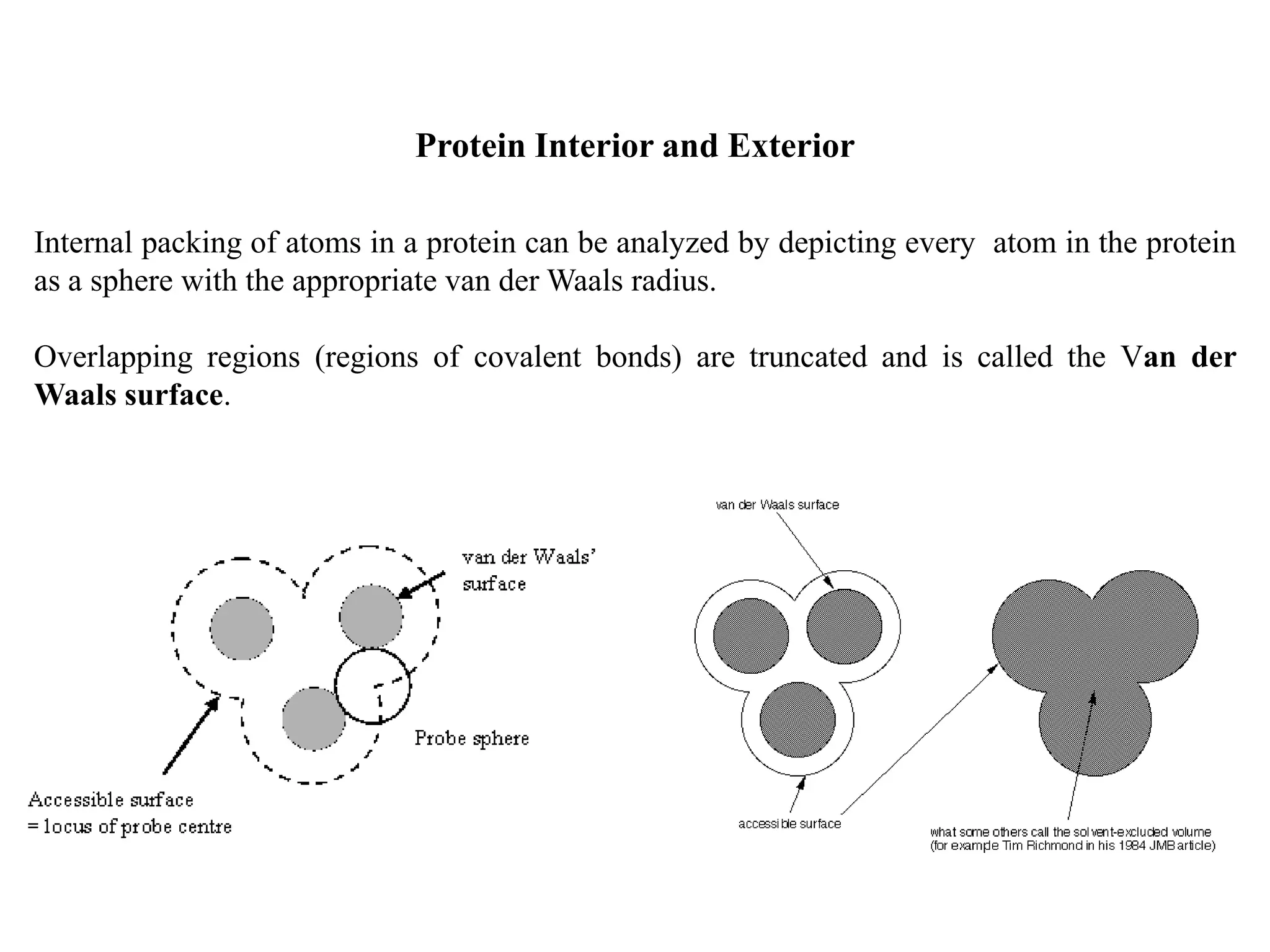
1. Non-covalent interactions like hydrogen bonds, ionic bonds, van der Waals forces, and hydrophobic interactions stabilize protein structure through weak but numerous attractive forces.
2. These interactions are weak but important because they allow proteins to dynamically change shape while maintaining overall structure, which enables biochemical reactions and functions.
3. The primary non-covalent attractive forces in macromolecules are electrostatic or ionic bonds, hydrogen bonds, van der Waals forces, and hydrophobic interactions, with electrostatic being the strongest.