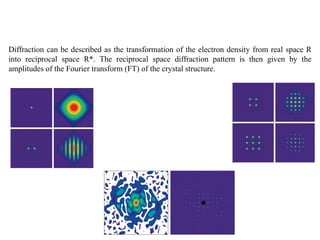
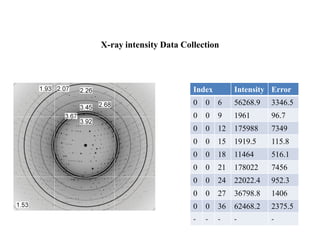
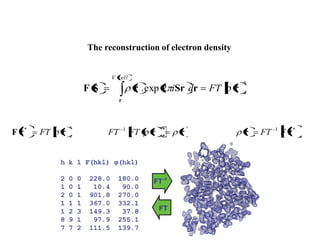
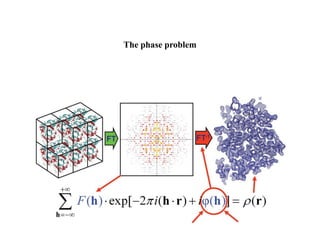
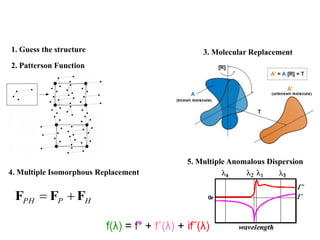
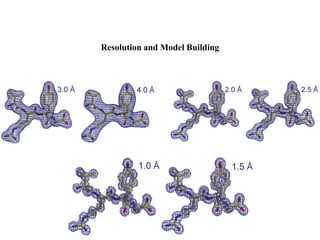
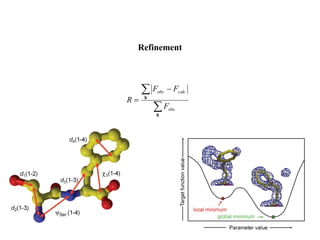
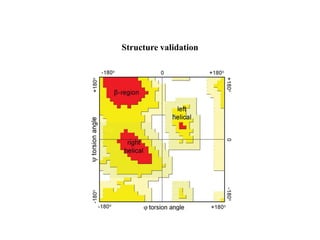
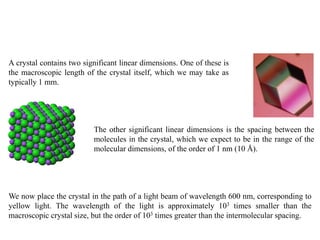
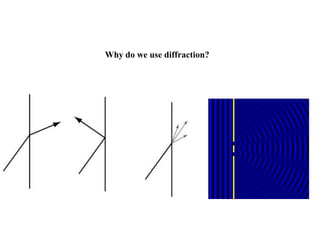
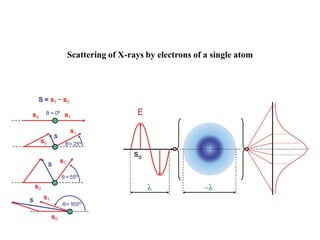
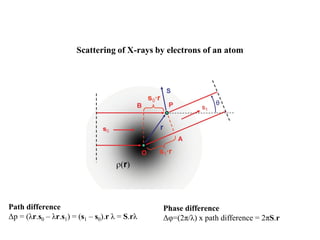
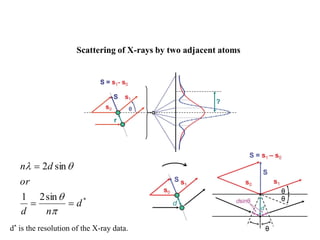
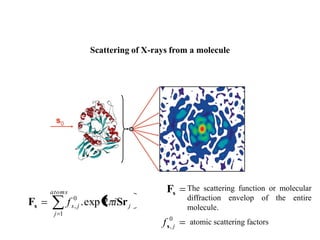
X-ray crystallography uses X-rays to determine the atomic and molecular structure of crystals. X-rays have wavelengths small enough (~0.1nm) to probe the distances between atoms in crystals. When X-rays hit a crystal, they cause the electrons of individual atoms to scatter the X-rays in all directions. The scattered X-rays interfere with one another, producing a diffraction pattern that can be used to reveal the crystal structure. Crystals are required because they produce repeated patterns that amplify the X-ray scattering, making it possible to determine atomic positions.





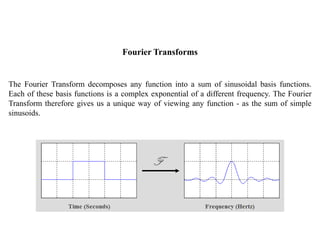
![Fourier Transforms
Convolution Theorem:
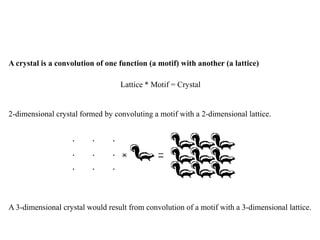
Convolution: take one function, f(r), and put it down at every point of a second function, g(r);
f(r)*g(r). Here * is the convolution operator.
Convolution Theorem states: FT [f(r) * g(r)] = F(S) • G(S); that is, the Fourier transform of
one function convoluted with another is the same as the Fourier transform of the first
multiplied by the Fourier transform of the second (here we will set a convention; real space
functions and their coordinate symbols are expressed in lower case, e.g. f, g, and r, and their
Fourier transforms and frequency space coordinates are expressed in upper case, e.g. F, G, and
S). The converse is also true: F(S) * G(S) = FT [f(r)•g(r)].](https://image.slidesharecdn.com/bt63115x-raycrystallographydiffracton-140325231609-phpapp02/85/BT631-15-X-Ray_Crystallography_Diffracton-18-320.jpg)
![FT (motif) -- a continuous function -- no sharp discontinuities
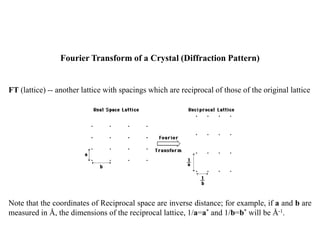
FT(crystal) = FT[(motif) * (lattice)] = FT(motif) . FT(lattice) = FT(motif) . (Reciprocal
Lattice).
Thus, the continuous Fourier transform of the motif is sampled at the points of the Reciprocal
lattice; the Fourier transform of the crystal is only non-zero at the points of the Reciprocal
lattice.](https://image.slidesharecdn.com/bt63115x-raycrystallographydiffracton-140325231609-phpapp02/85/BT631-15-X-Ray_Crystallography_Diffracton-21-320.jpg)