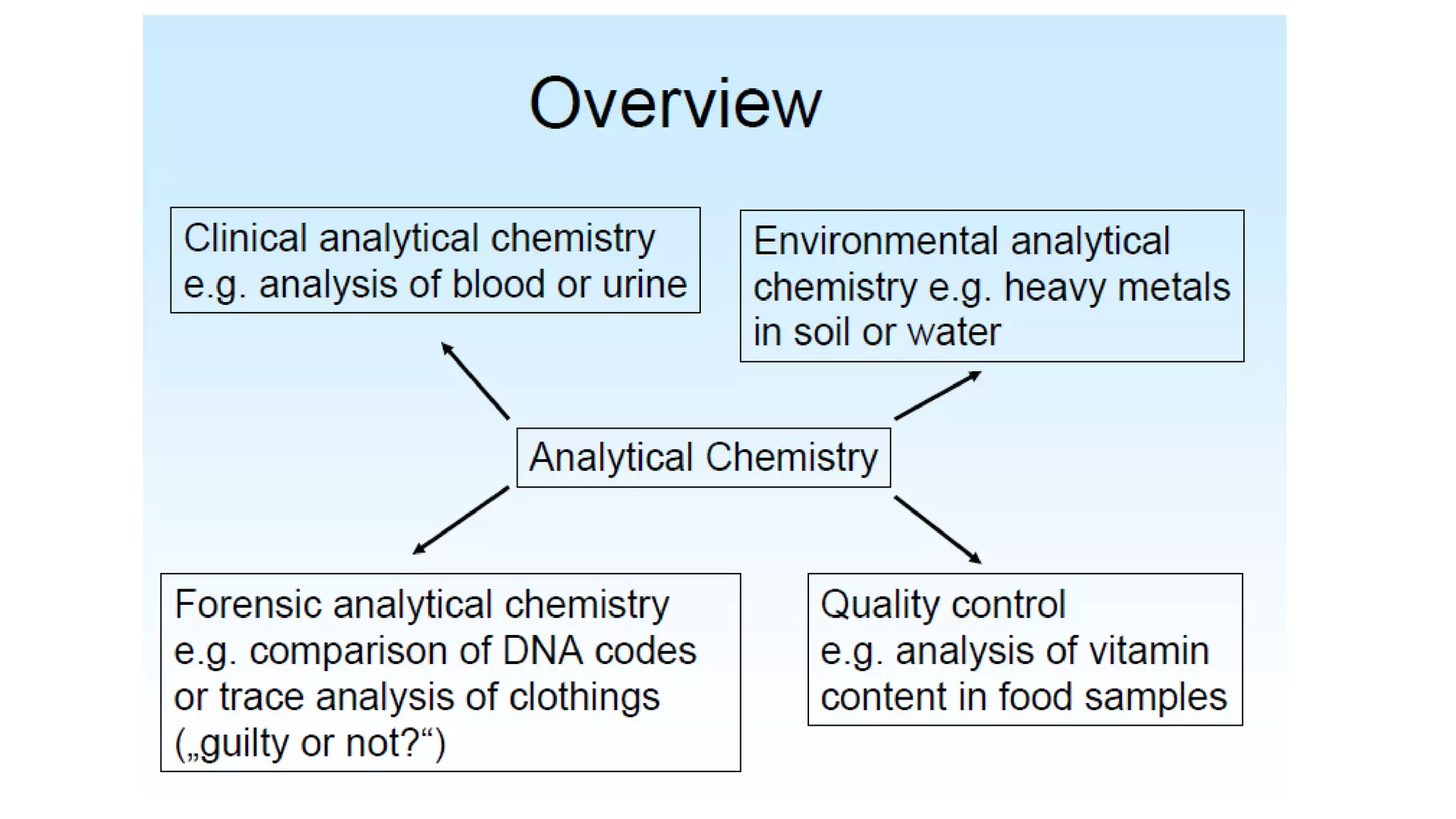
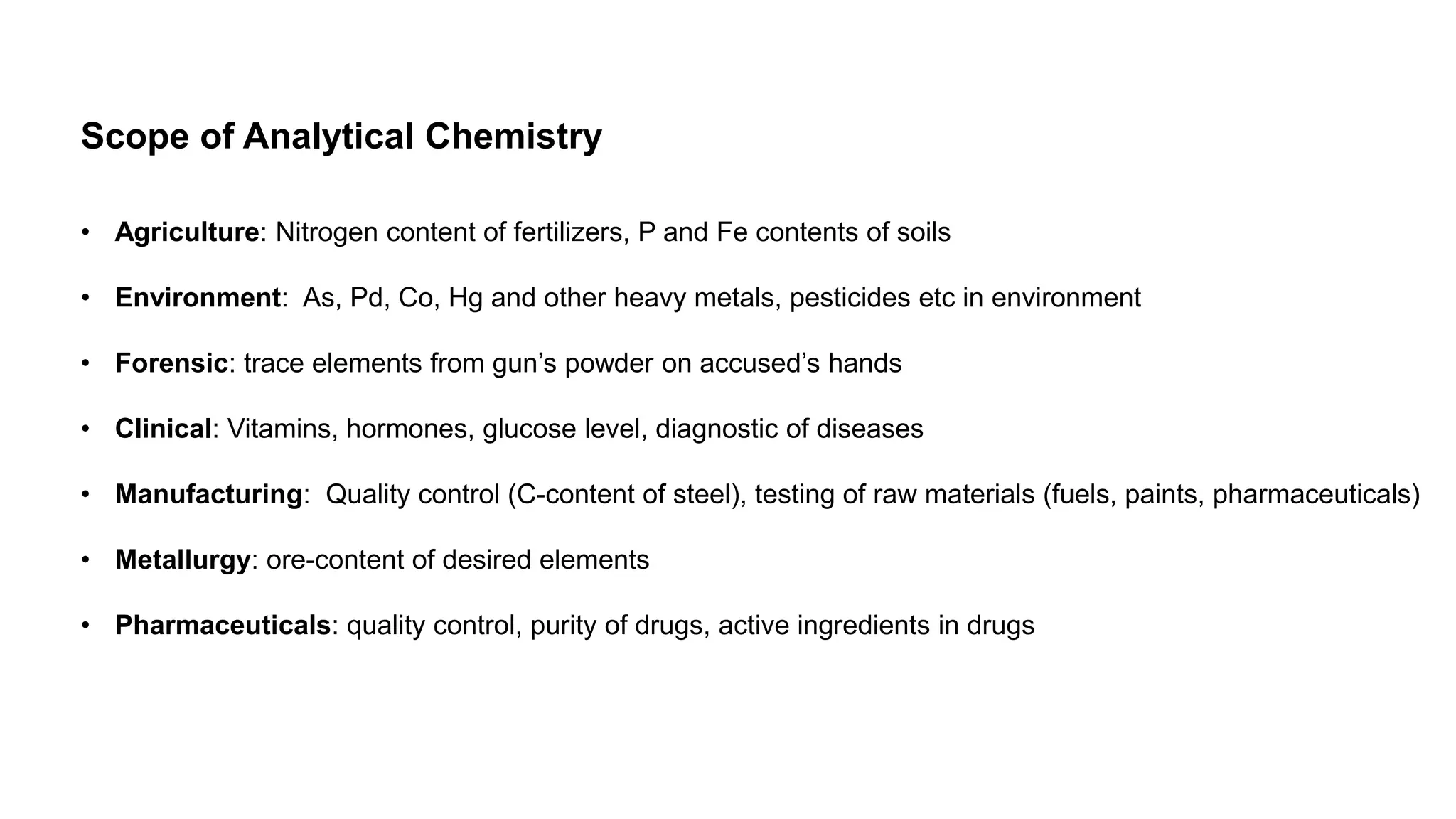
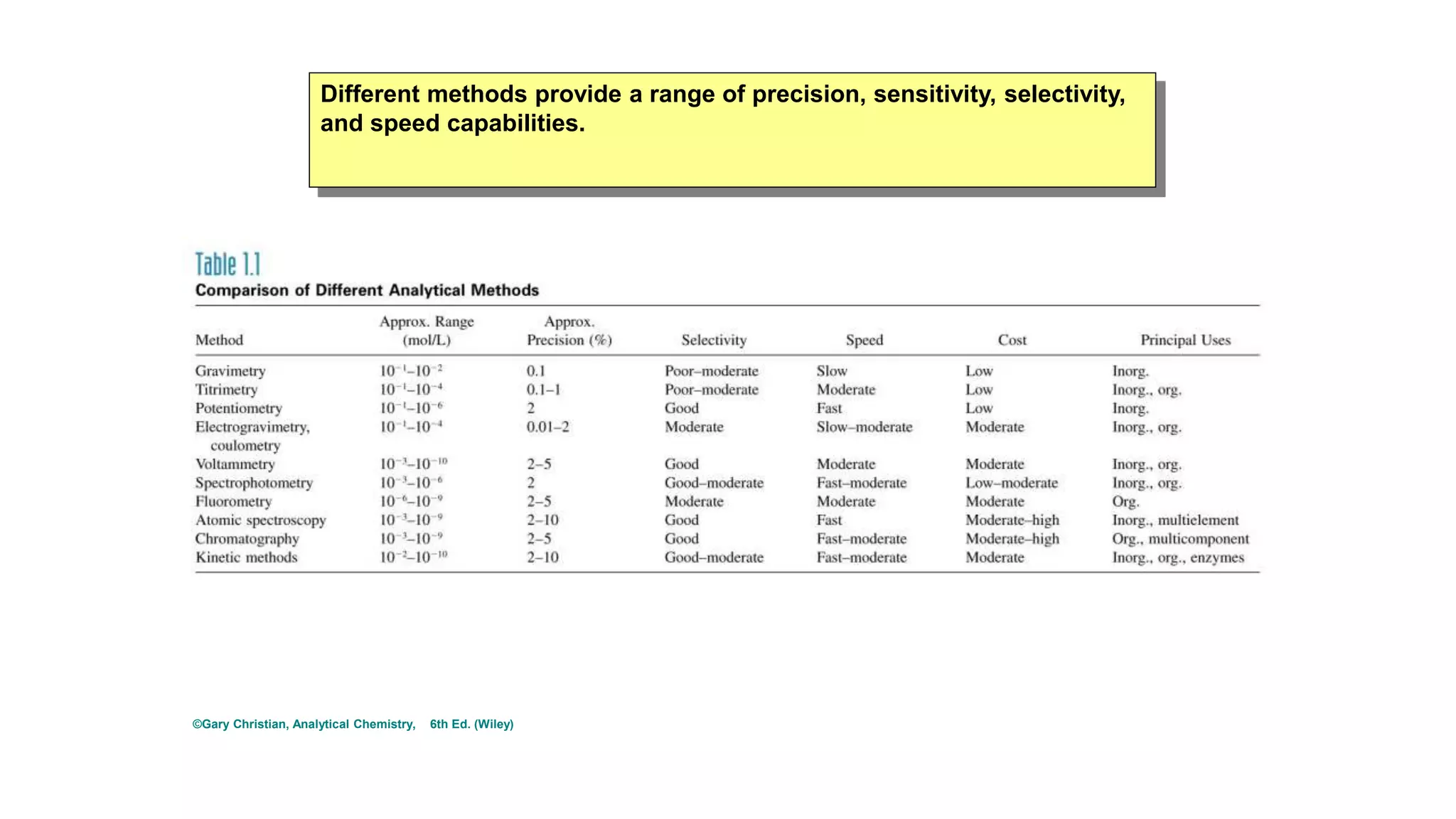
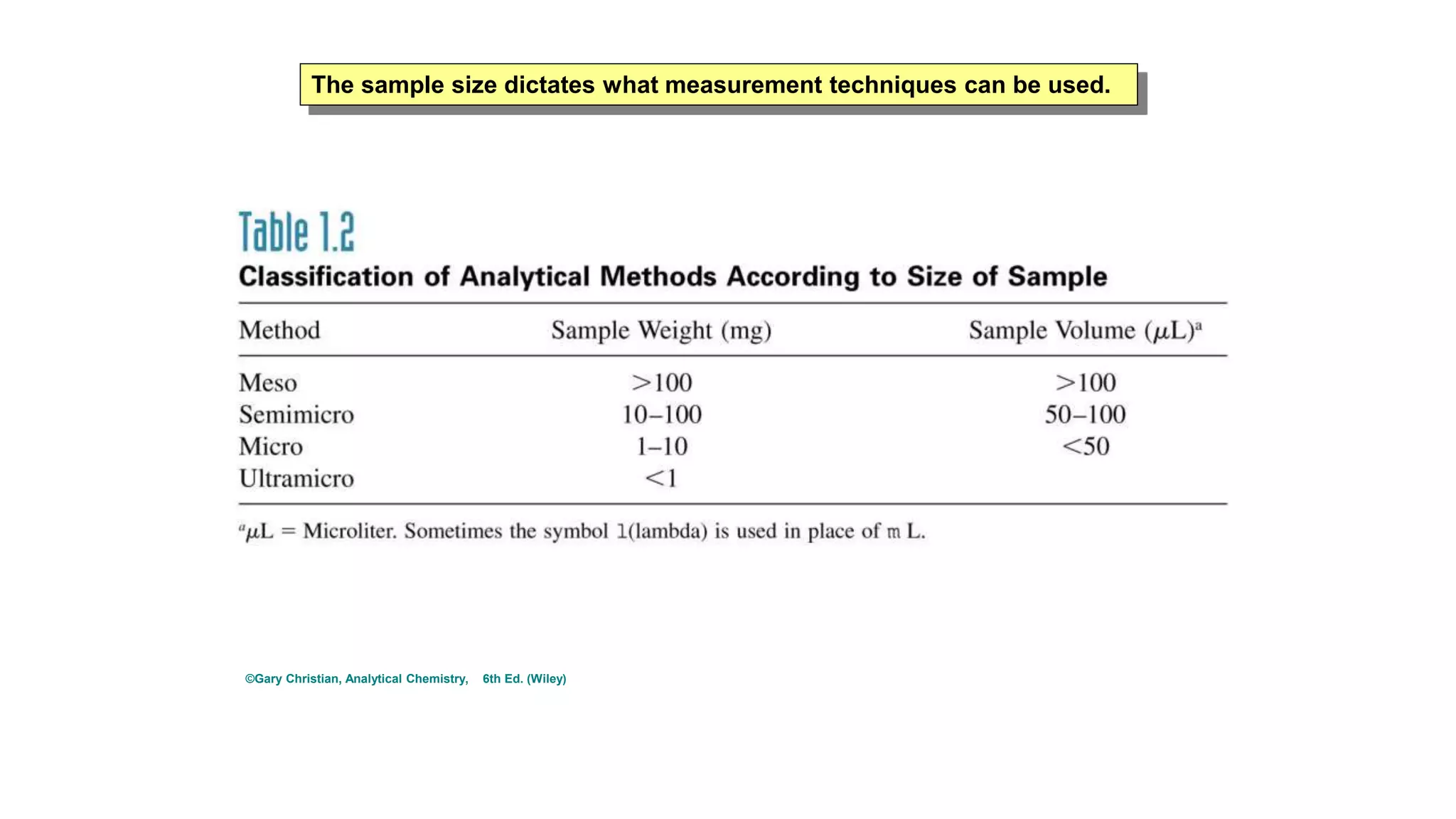
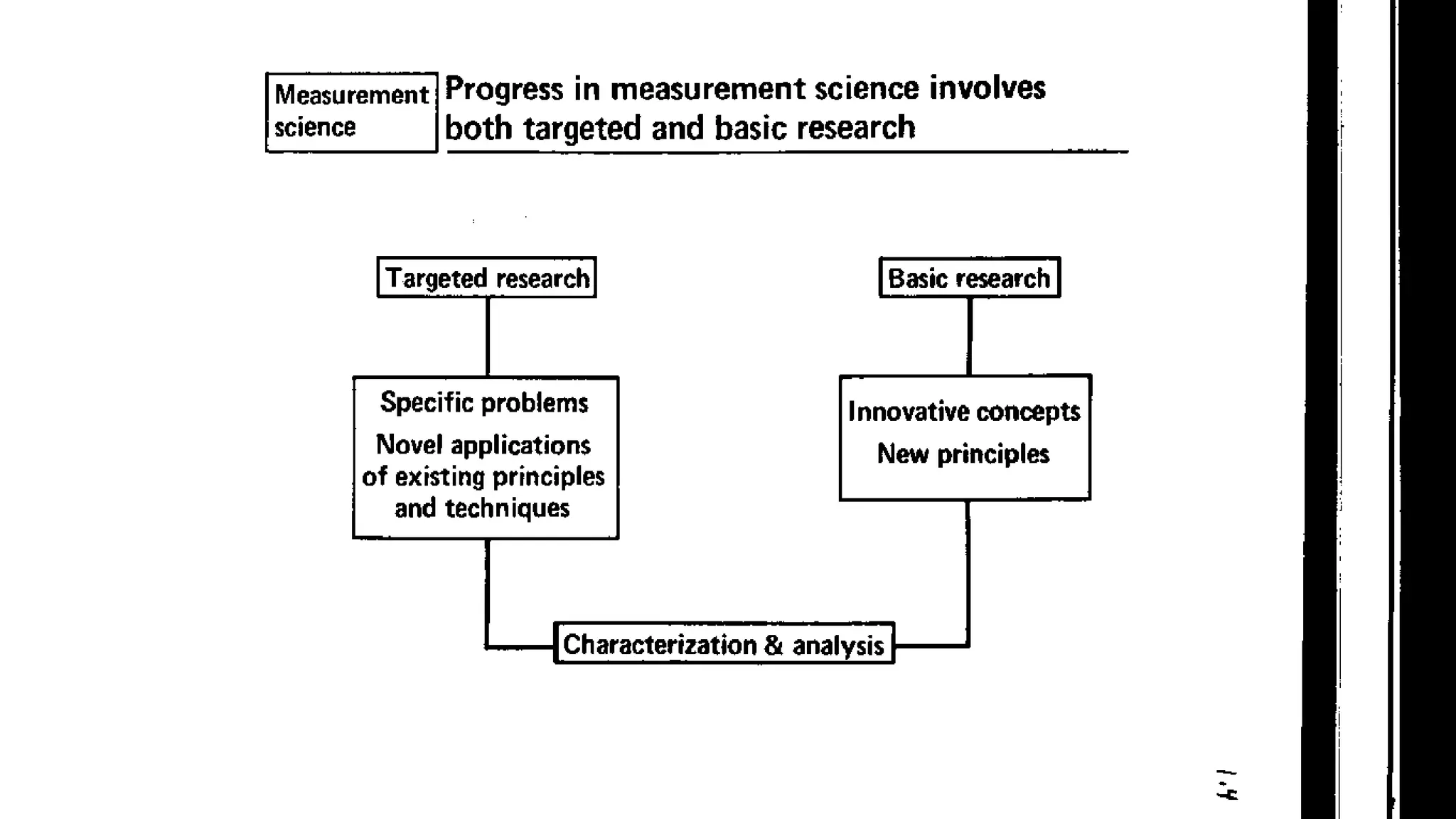
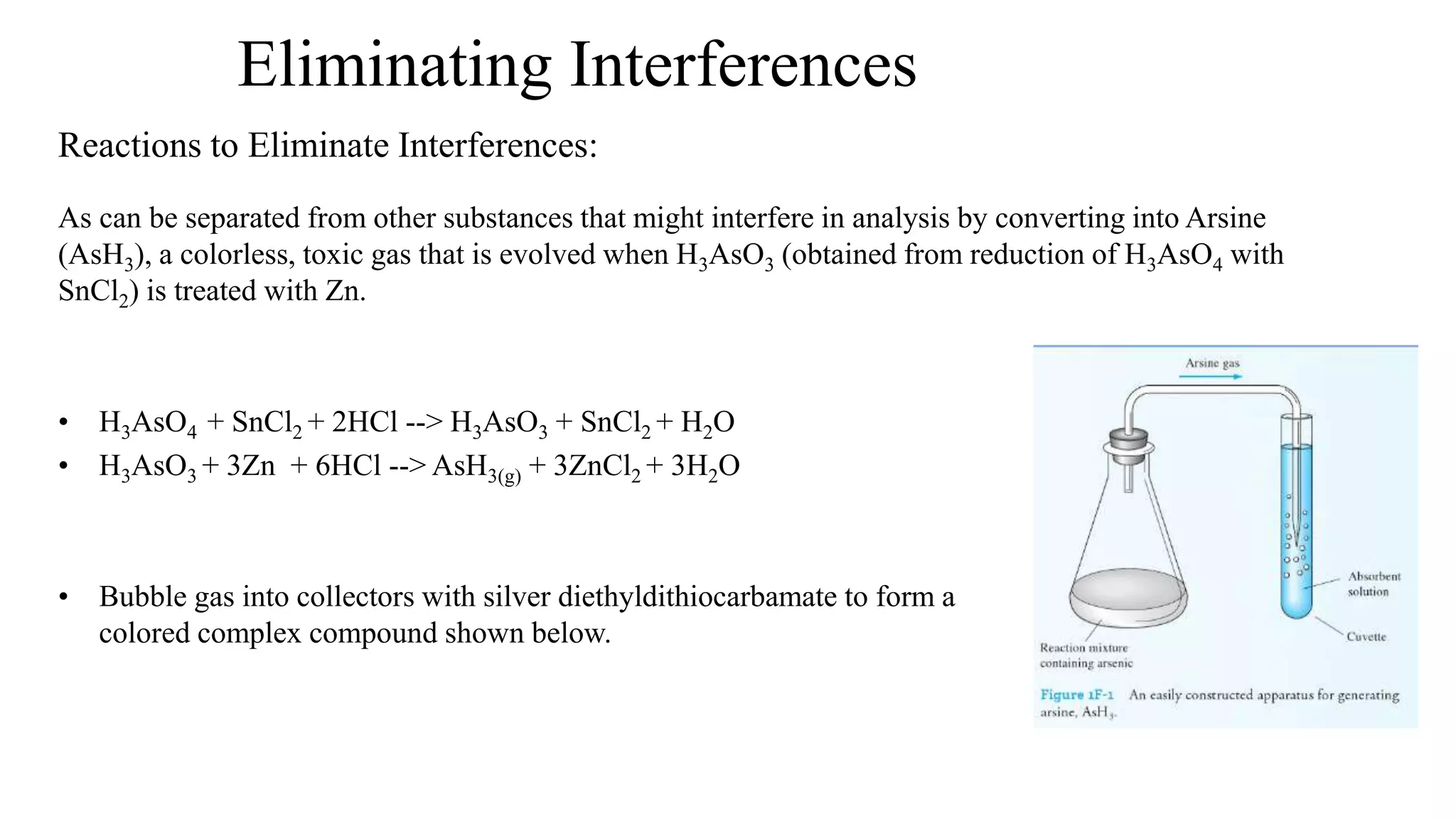
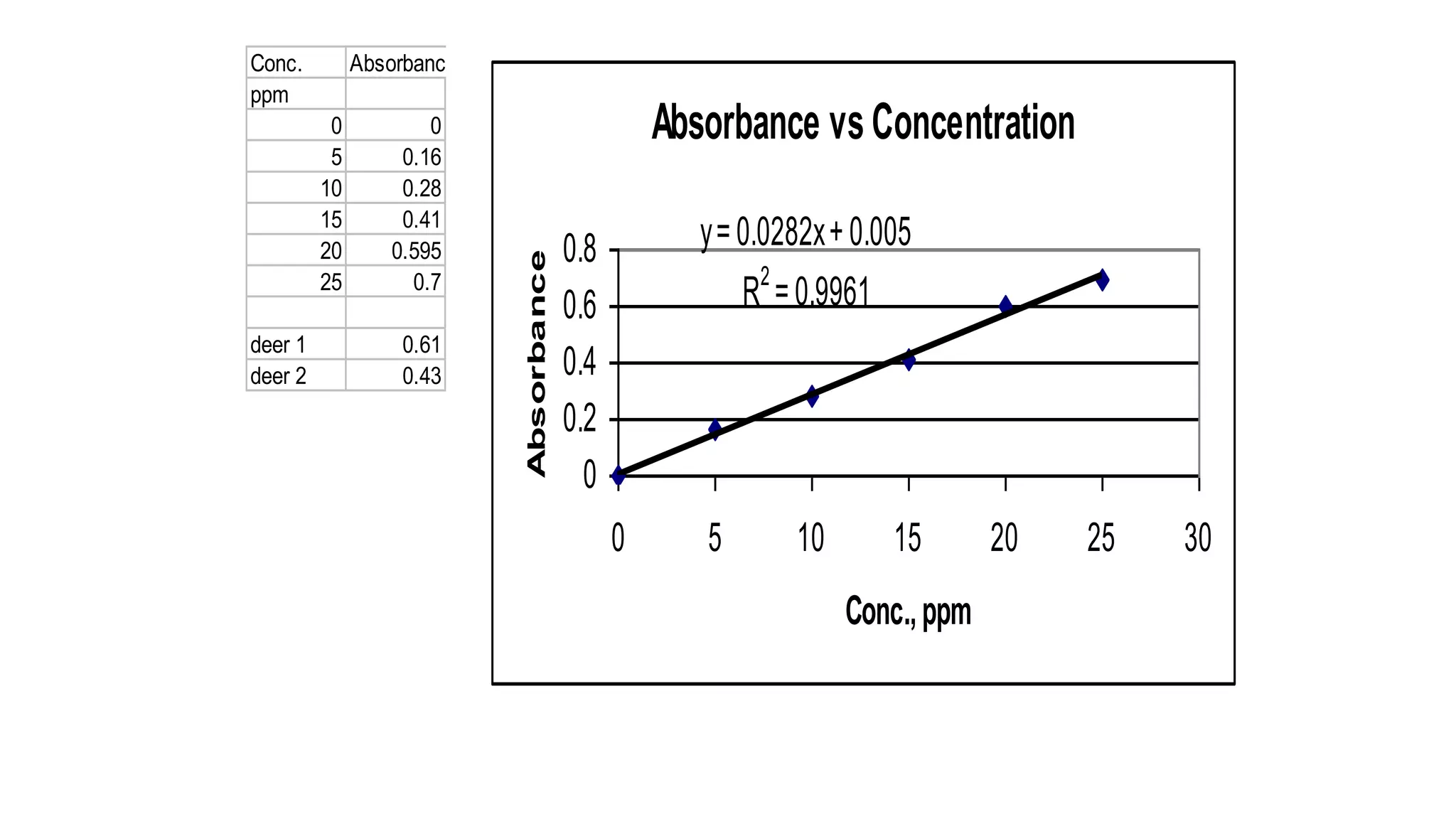
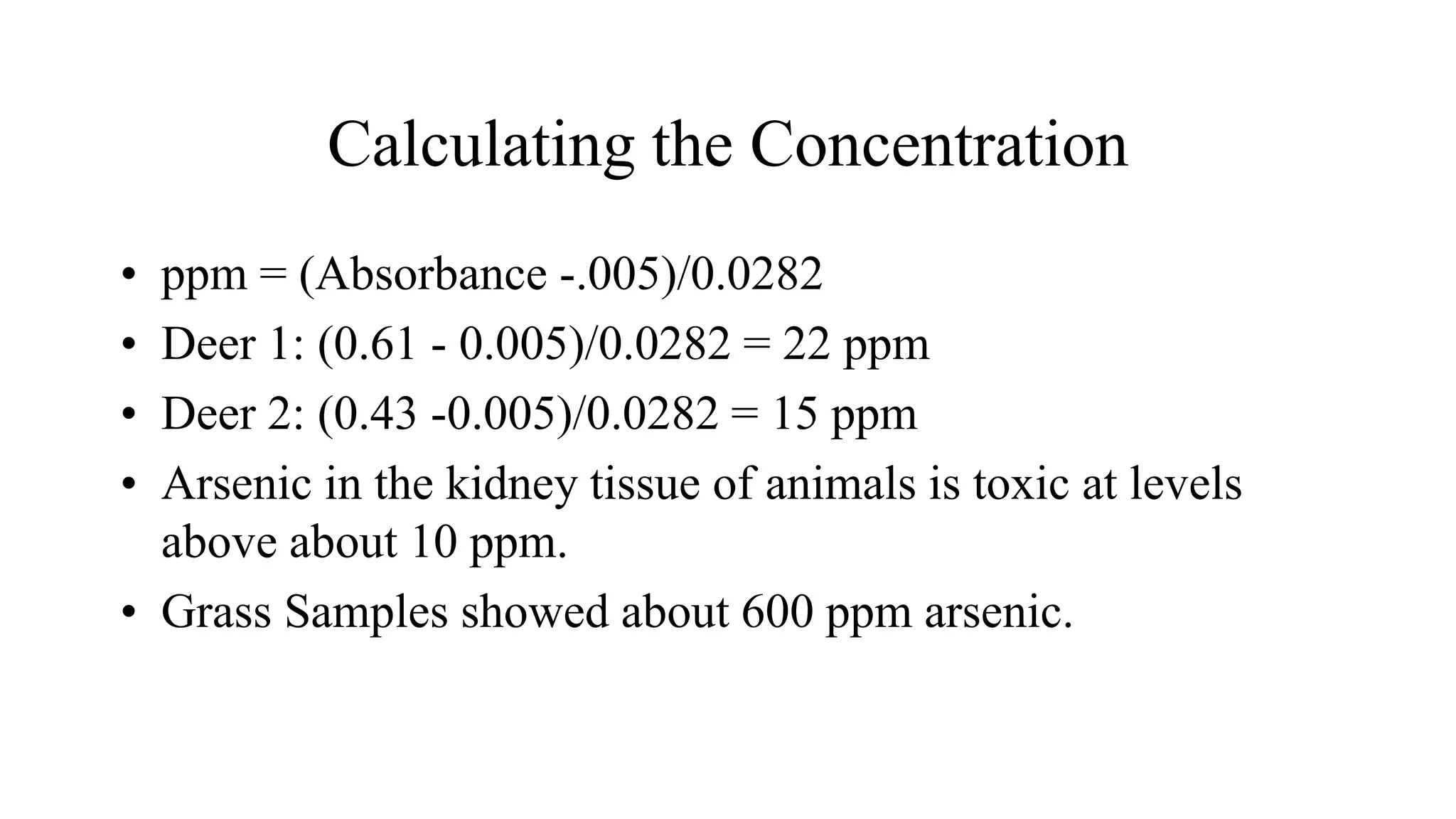
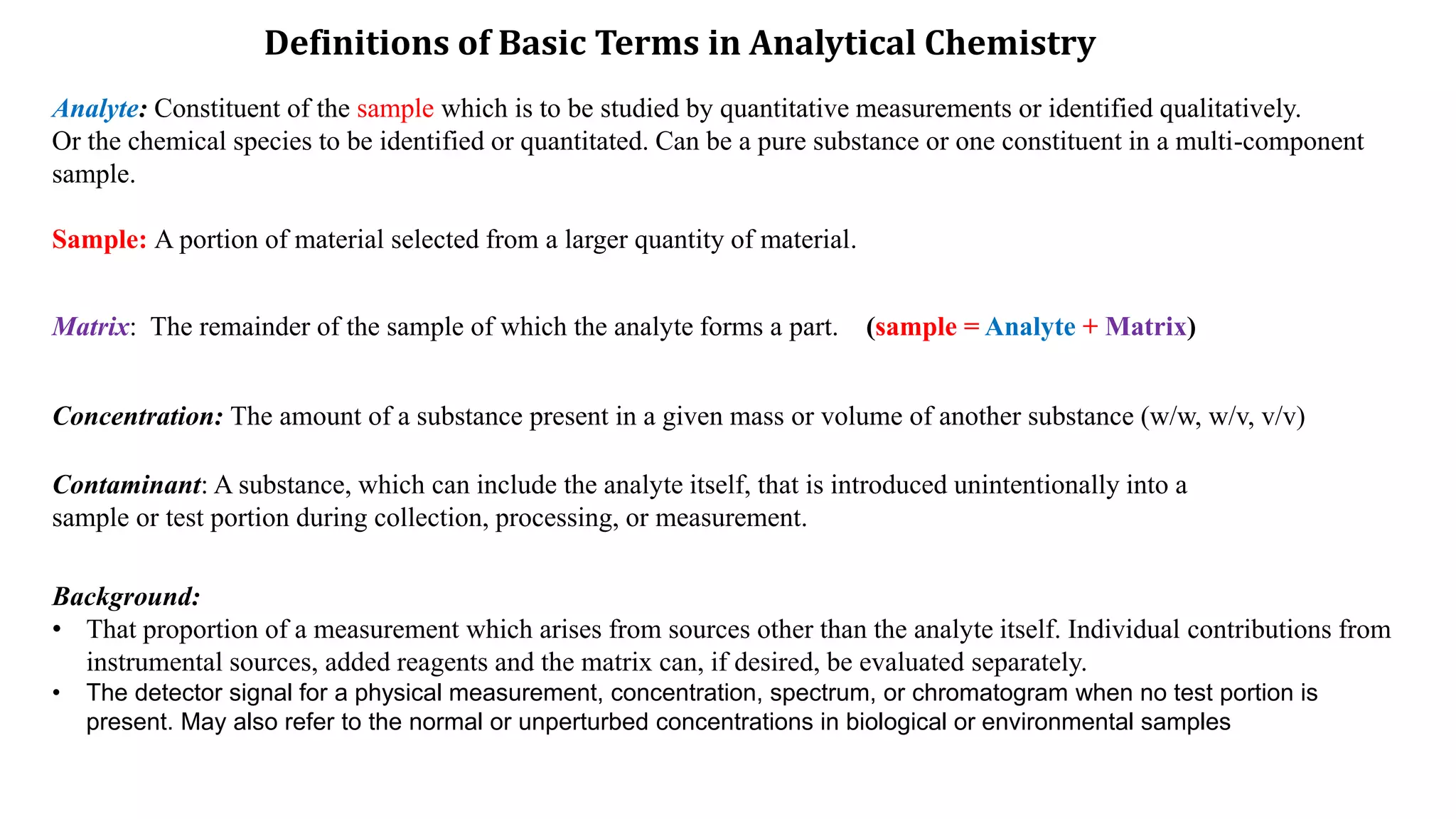
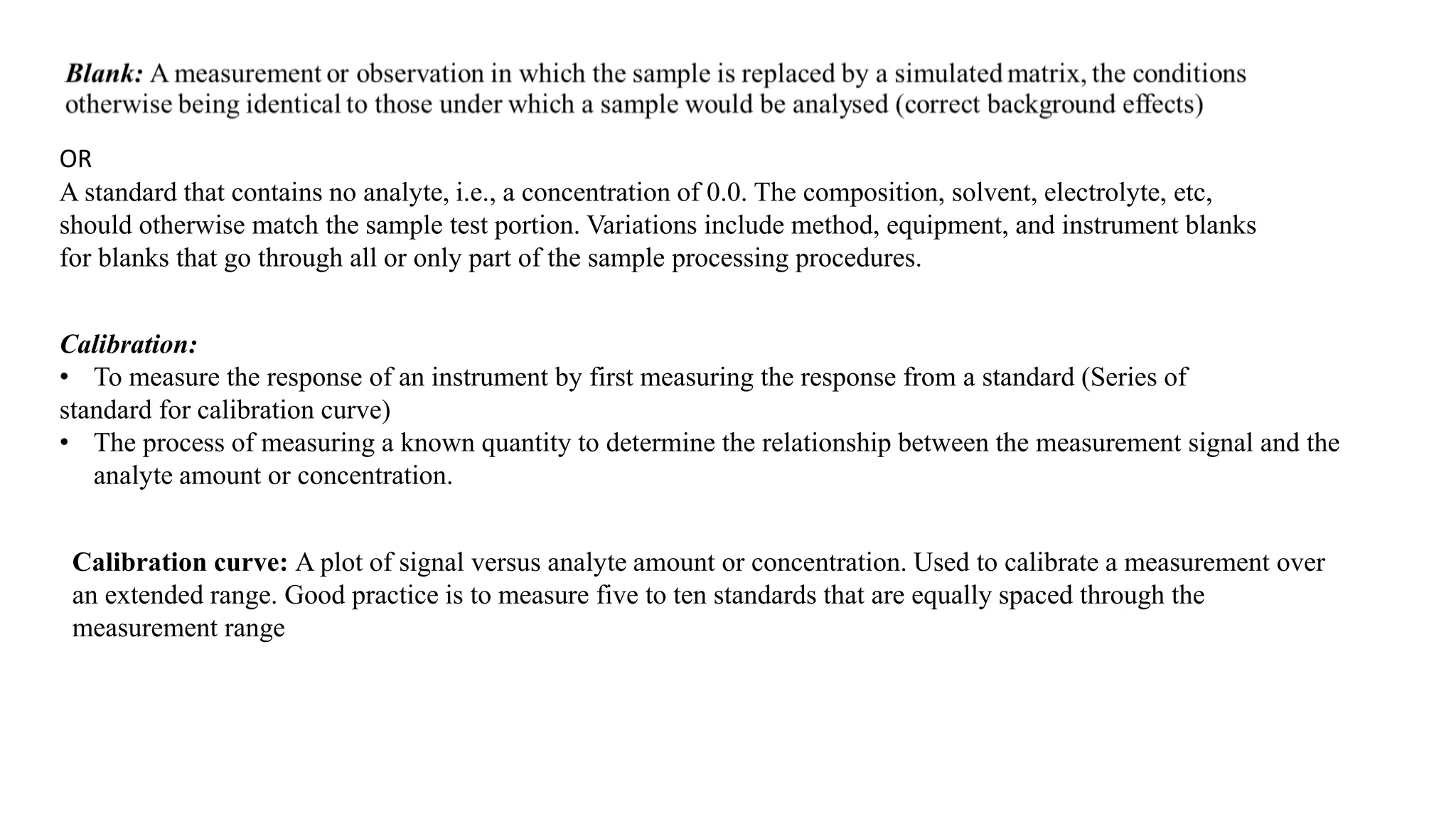
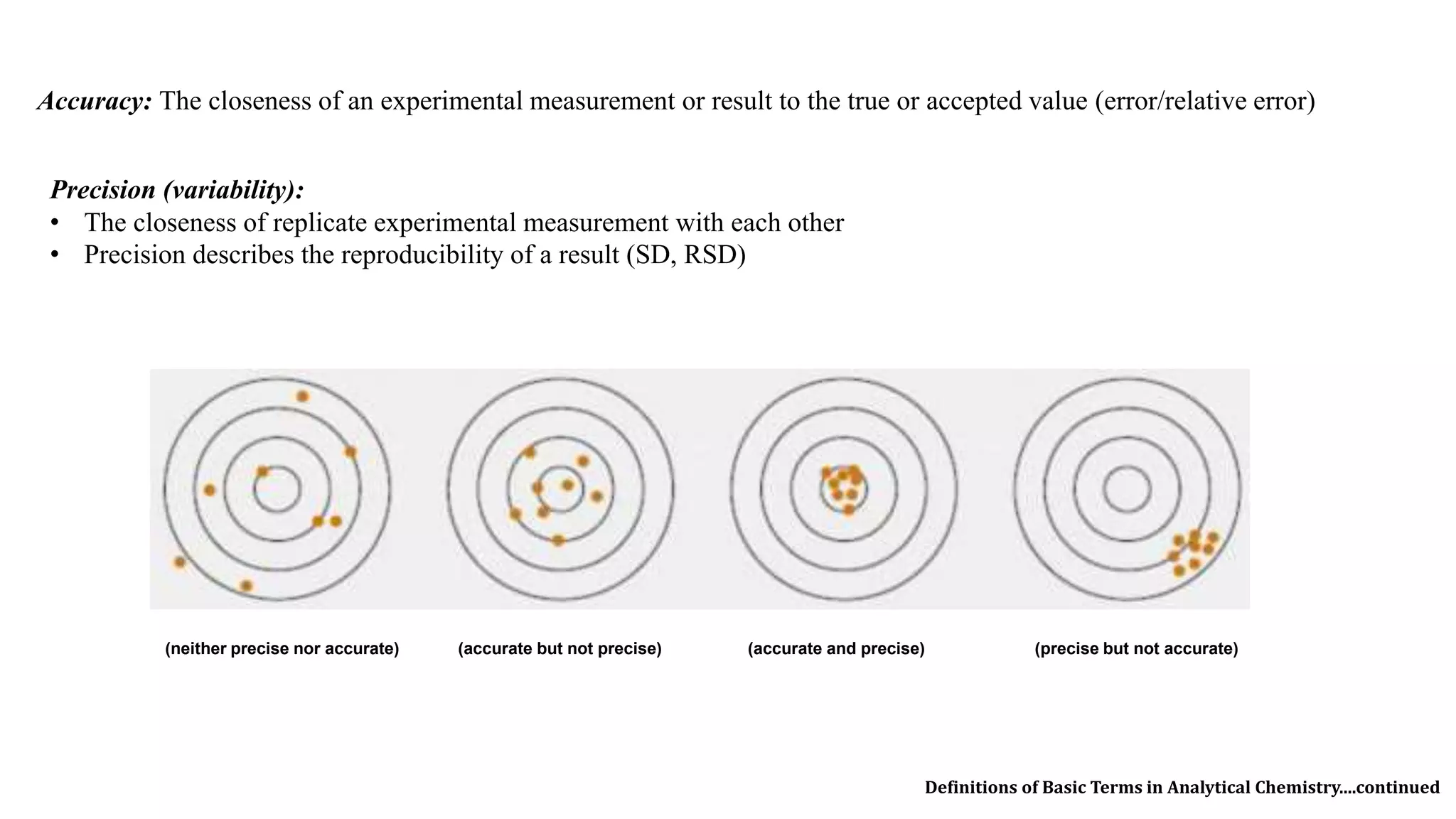
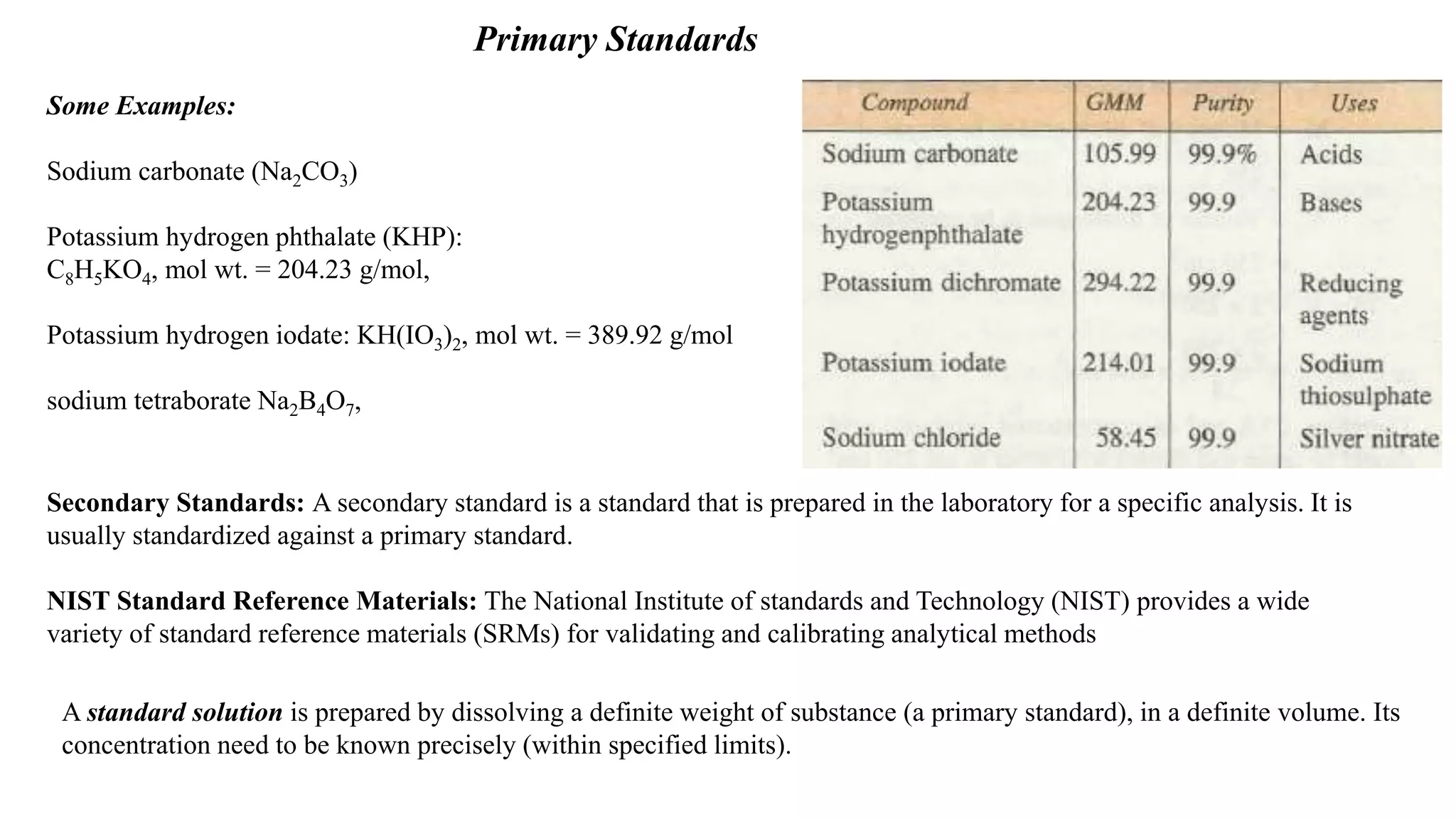
The document defines key terms used in analytical chemistry, including analyte, sample, matrix, concentration, calibration curve, accuracy, precision, interference, standards, and more. It also provides examples of primary standards such as sodium carbonate and potassium hydrogen phthalate. Additionally, it discusses the scope of analytical chemistry and its role in fields like agriculture, environment, forensics, manufacturing, and more. The document aims to familiarize readers with fundamental concepts in analytical chemistry.

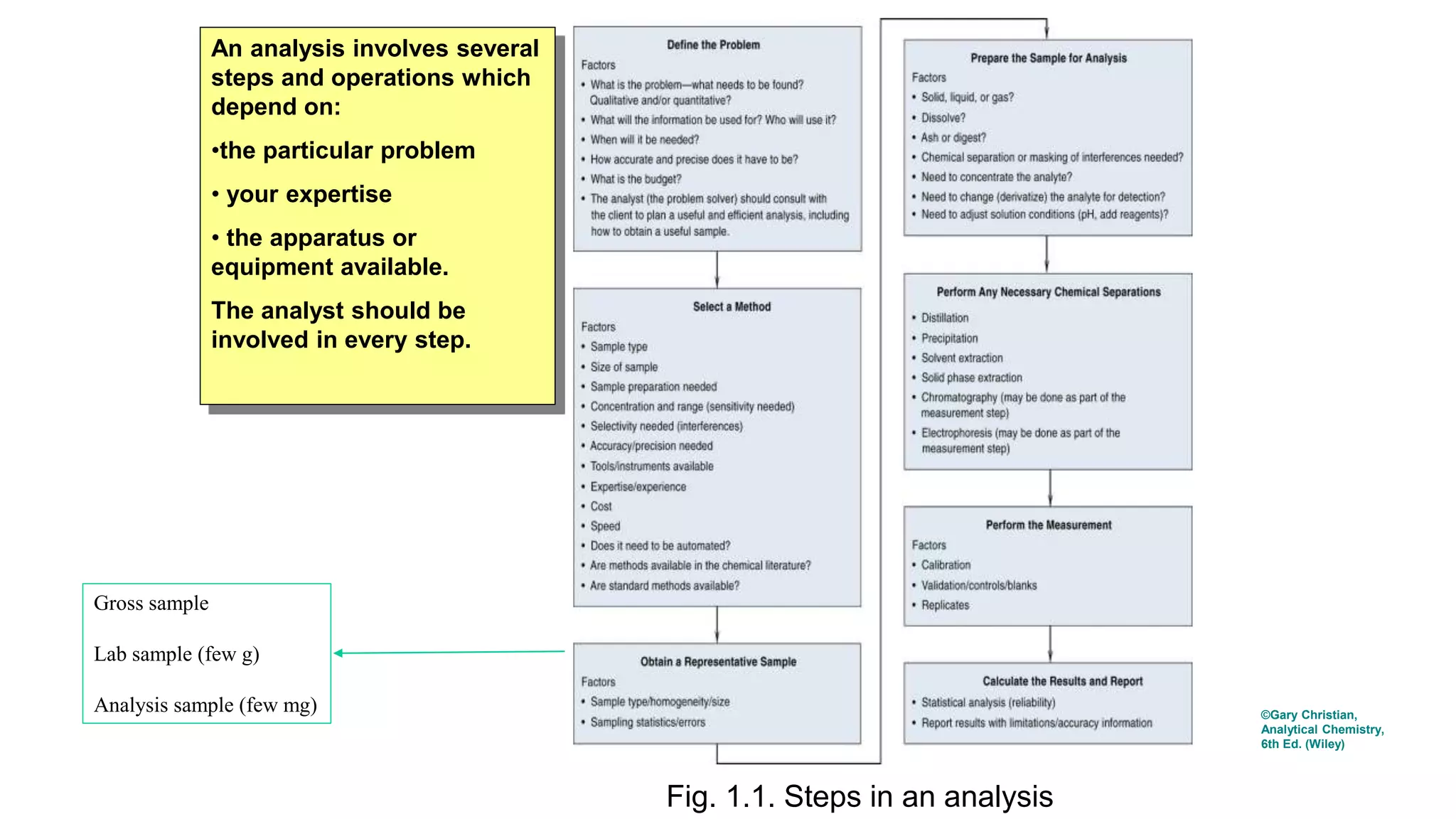
![Method development: Determining the experimental conditions for sample collection, preparation, and measurement that
produce accurate and repeatable results.
Validation of Method:
• to validate the method by analyzing standards which have an accepted analyte content, and a matrix similar to that of
the sample.
• Performing control experiments to verify the accuracy, sensitivity, specificity, and reproducibility of test methods.
Signal : The detector output that is displayed or recorded.
Noise: Random fluctuations in the signal. Usually quantified using the standard deviation of multiple measurements of a blank.
Selectivity: The ability of a method or instrument to measure an analyte in the presence of other constituents of the sample
or test portion. [Pure & Appl.Chem. 2001, 73(8), 1381–1386.]
Sensitivity: The change in the response from an analyte relative to a small variation in the amount being determined. The
sensitivity is equal to the slope of the calibration curve (the change in detector signal versus the change in amount of
analyte), being constant if the curve is linear. In other words, it is the ability of a method to facilitate the detection or
determination of an analyte. A higher sensitivity may allow measurement of a lower analyte concentration, depending on
the signal-to-noise ratio.](https://image.slidesharecdn.com/class-i-msc-i-introdcution-analyticalchem-190212031911/75/B-S-4-Class-1-Introduction-to-analytical-chemistry-8-2048.jpg)