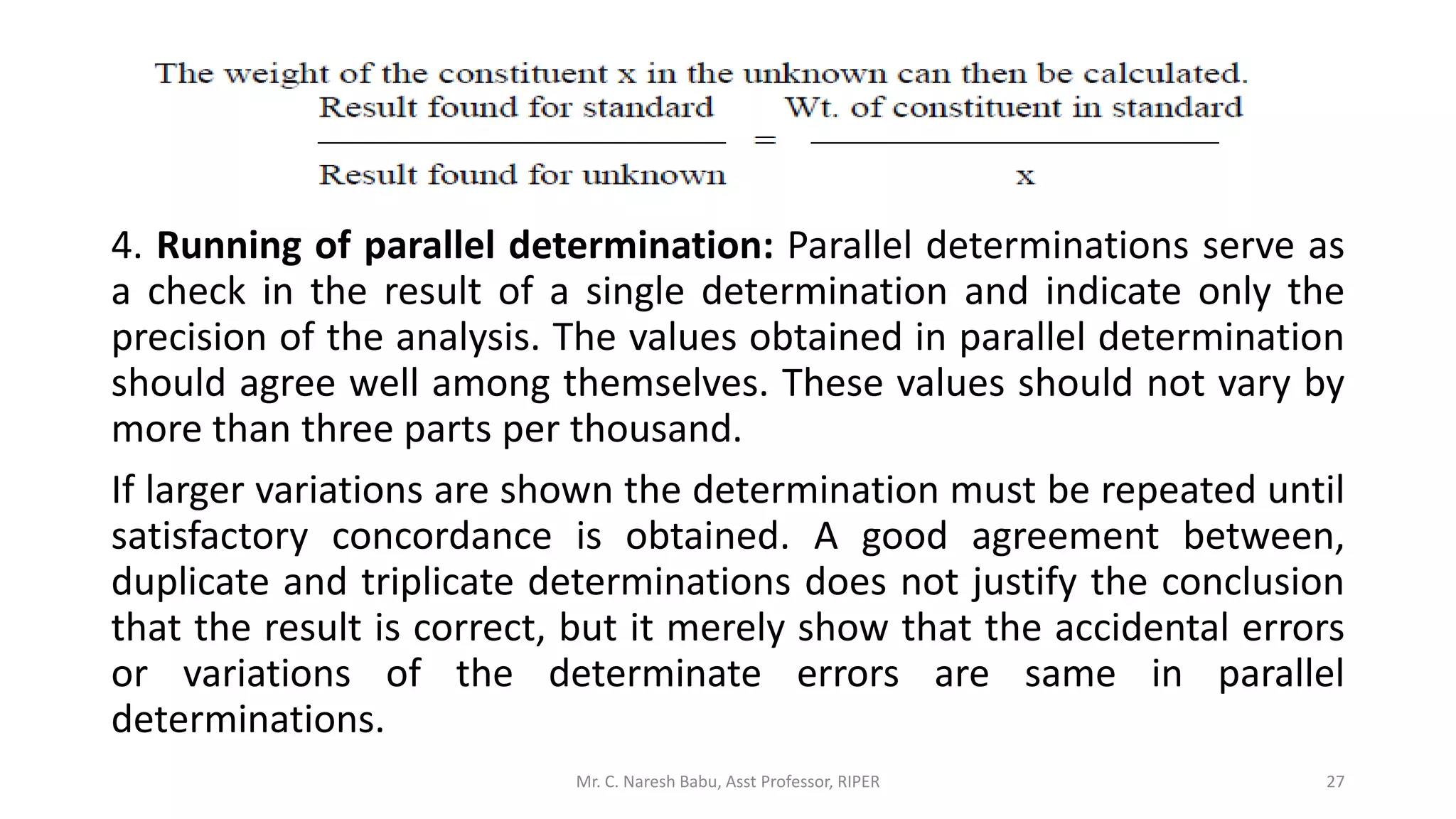
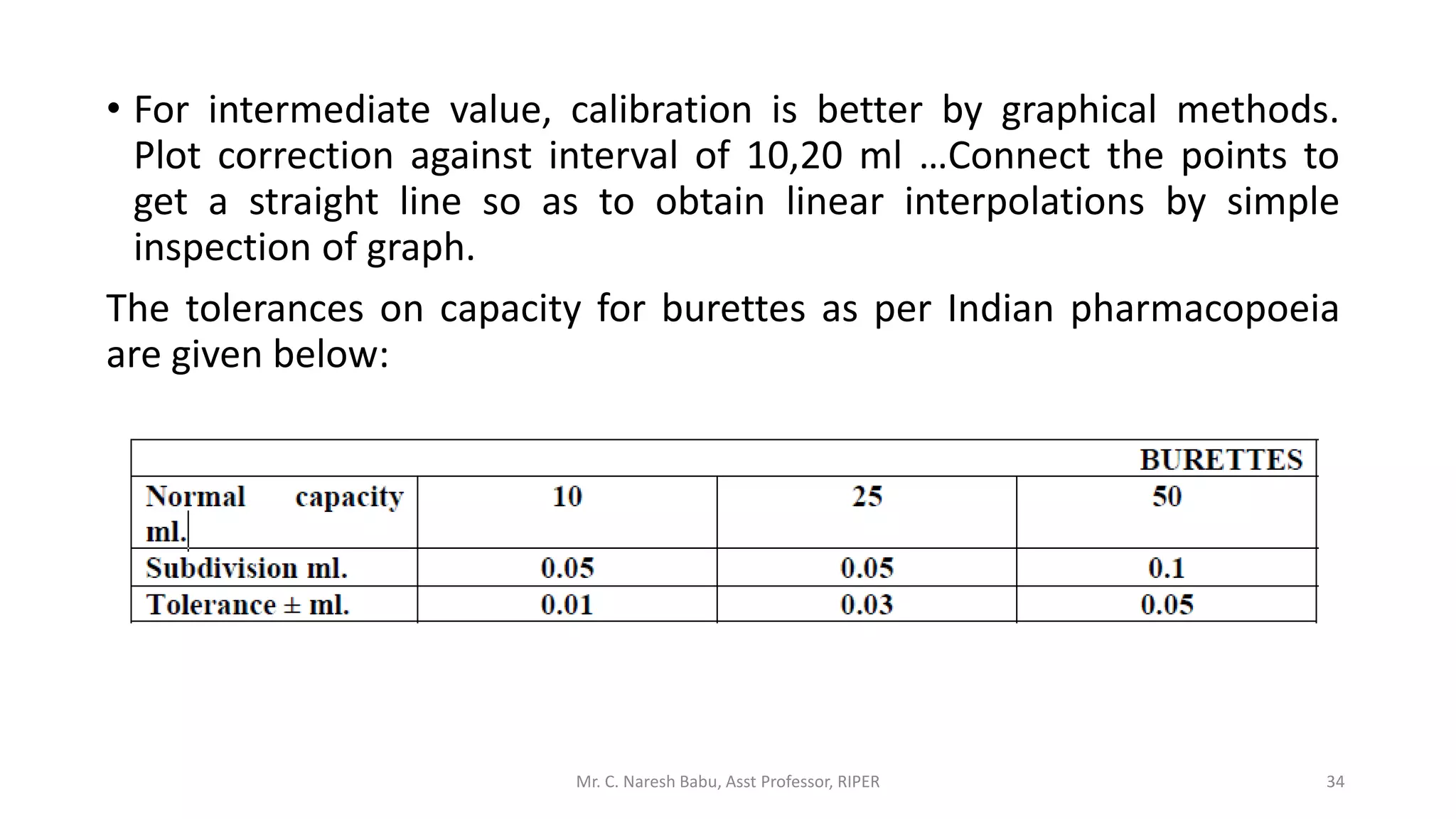
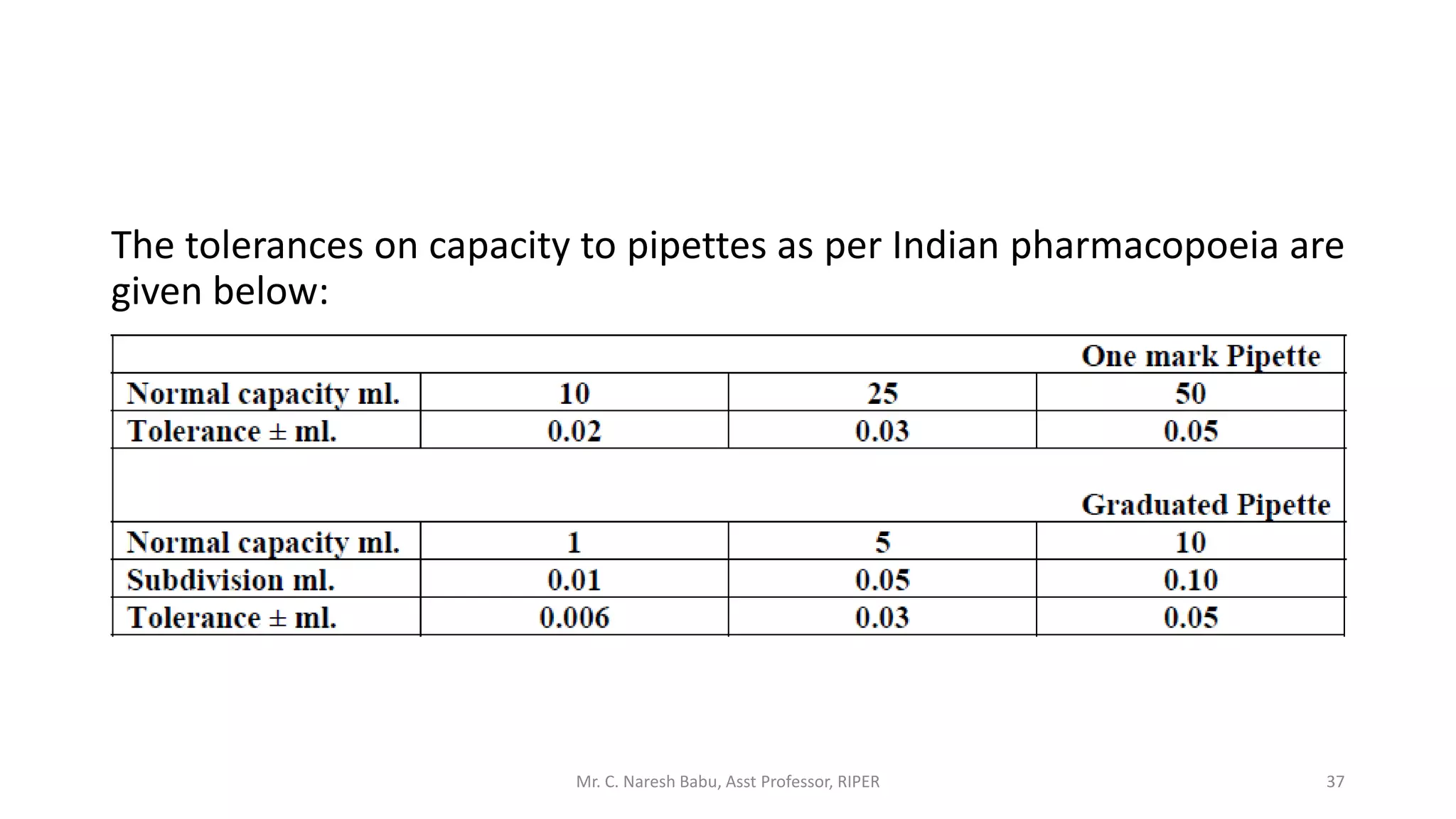
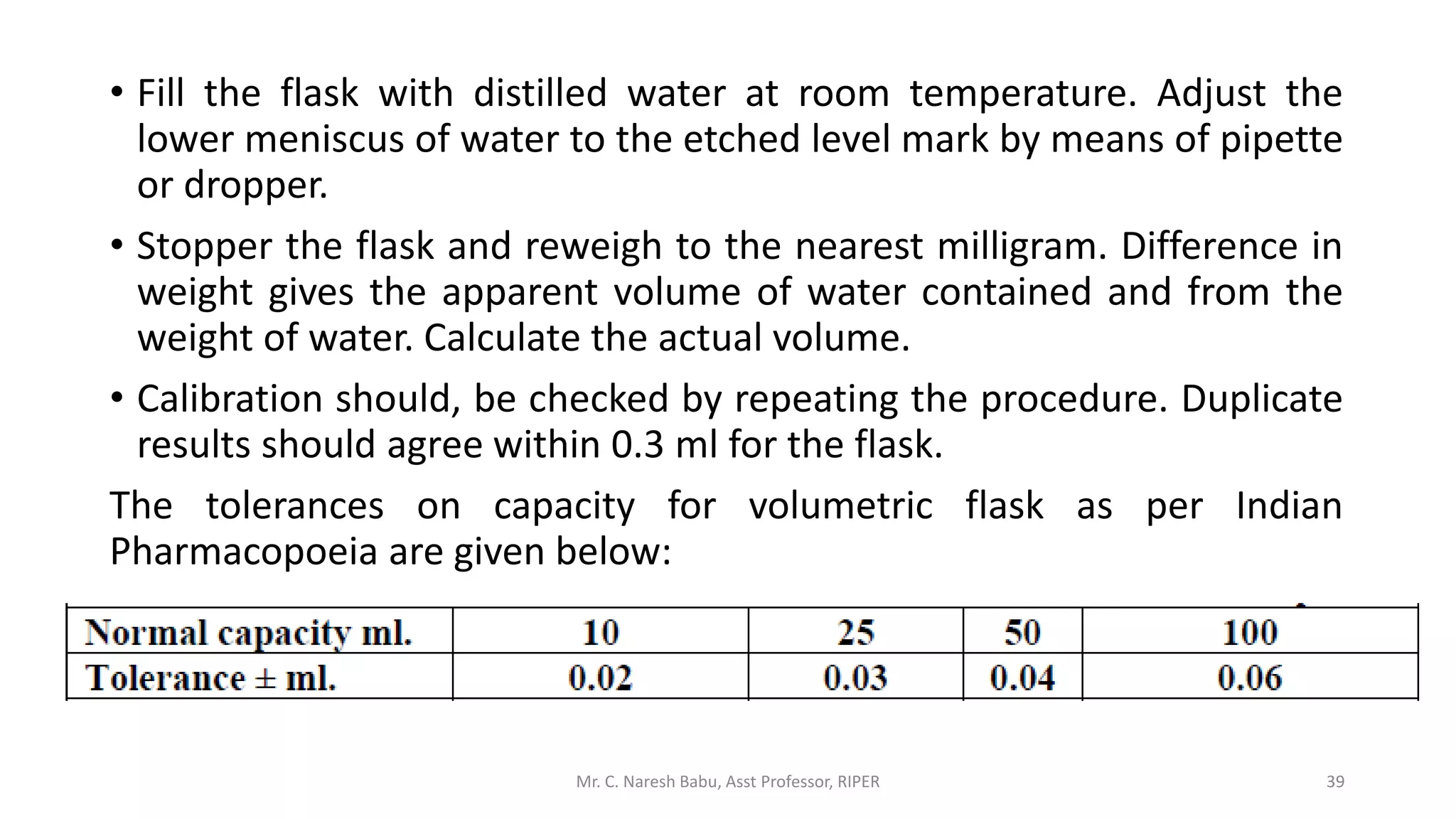
The document discusses the significance of analytical chemistry in the pharmaceutical industry, focusing on techniques for analyzing the composition, purity, and safety of drugs. It covers various types of chemical analysis, quality control practices, and methods used to minimize errors in measurements. The document also elaborates on significant figures, types of errors, and the importance of analytical methods in diagnosing diseases and ensuring compliance with regulations.