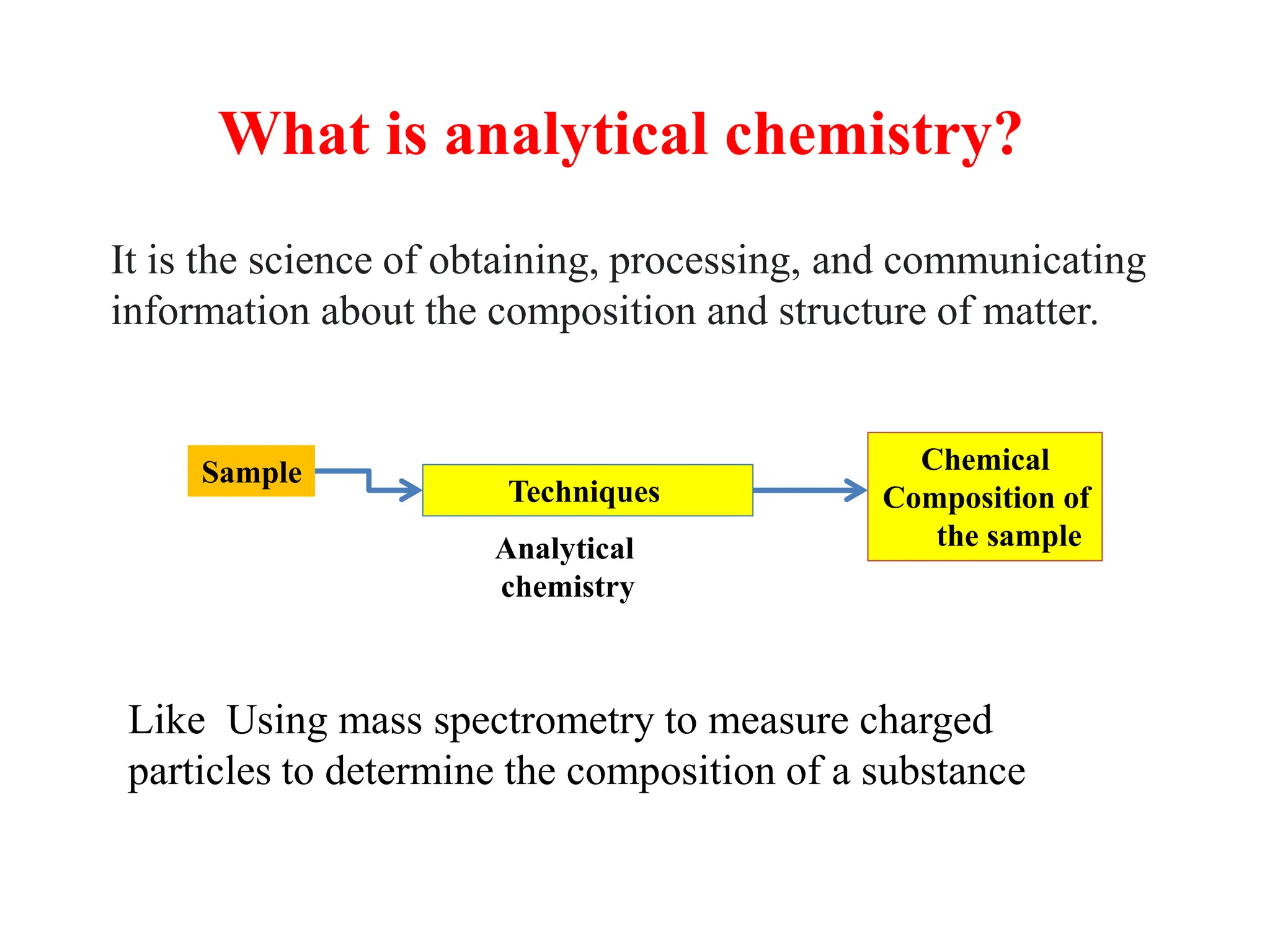
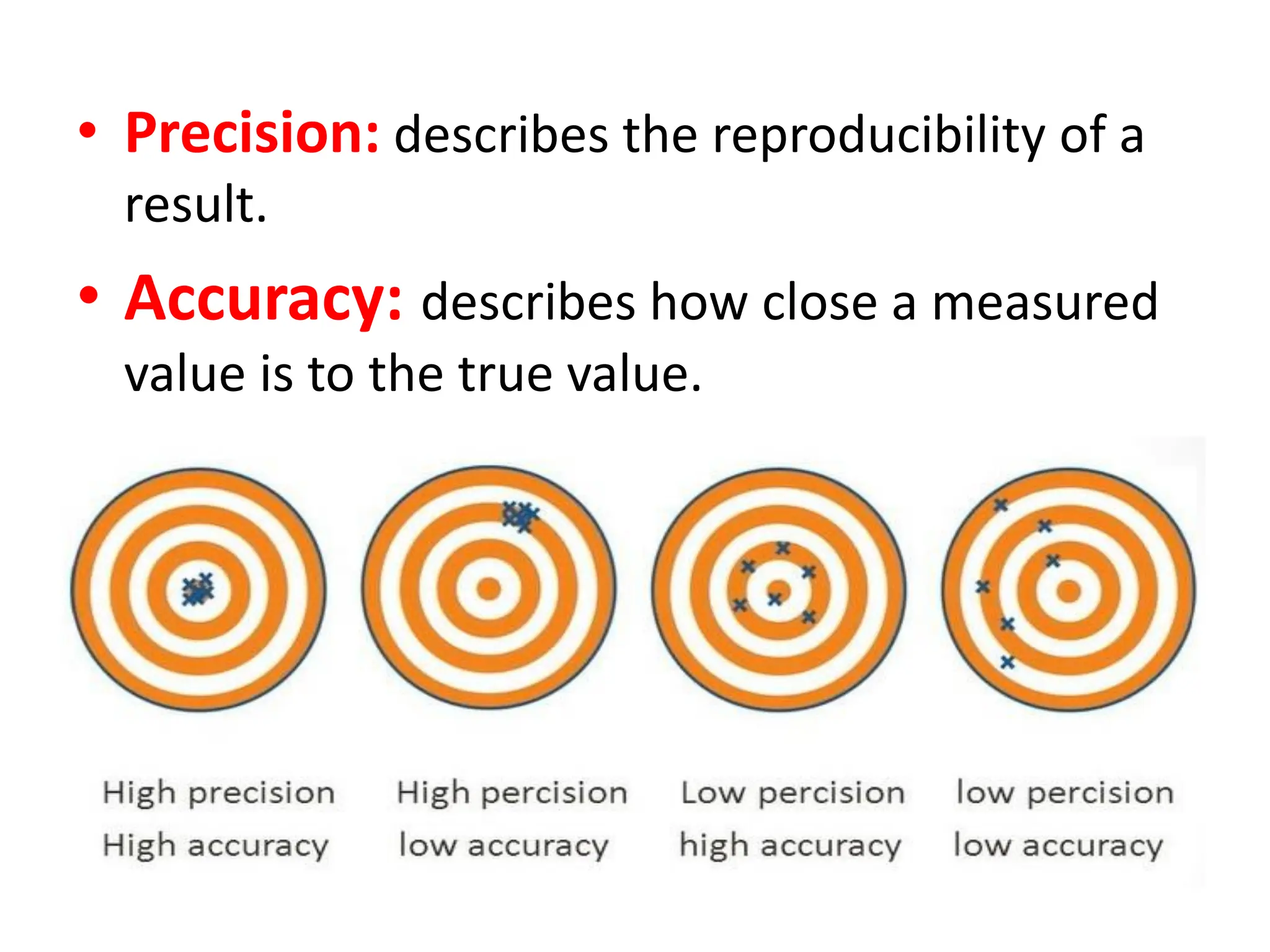
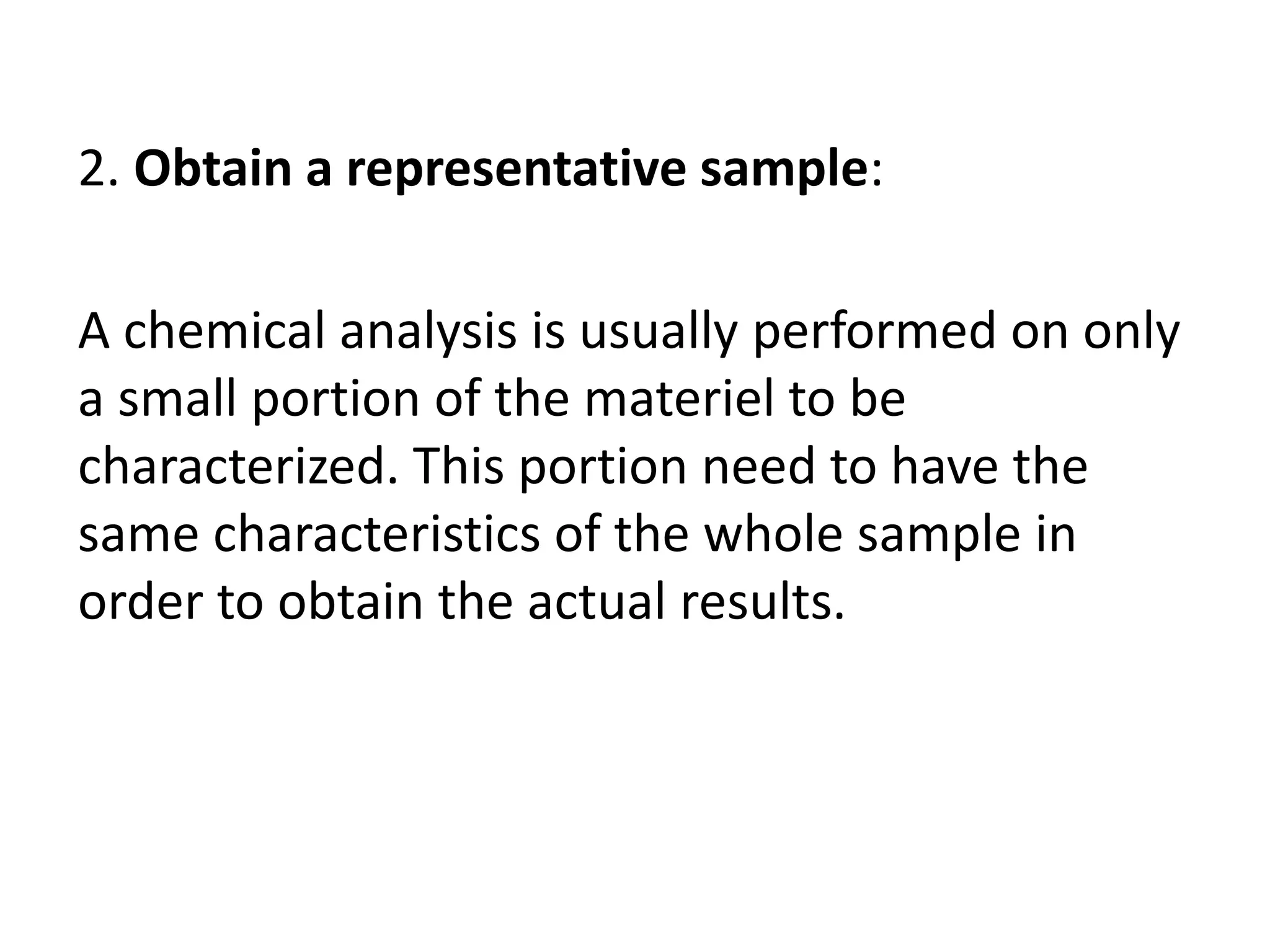
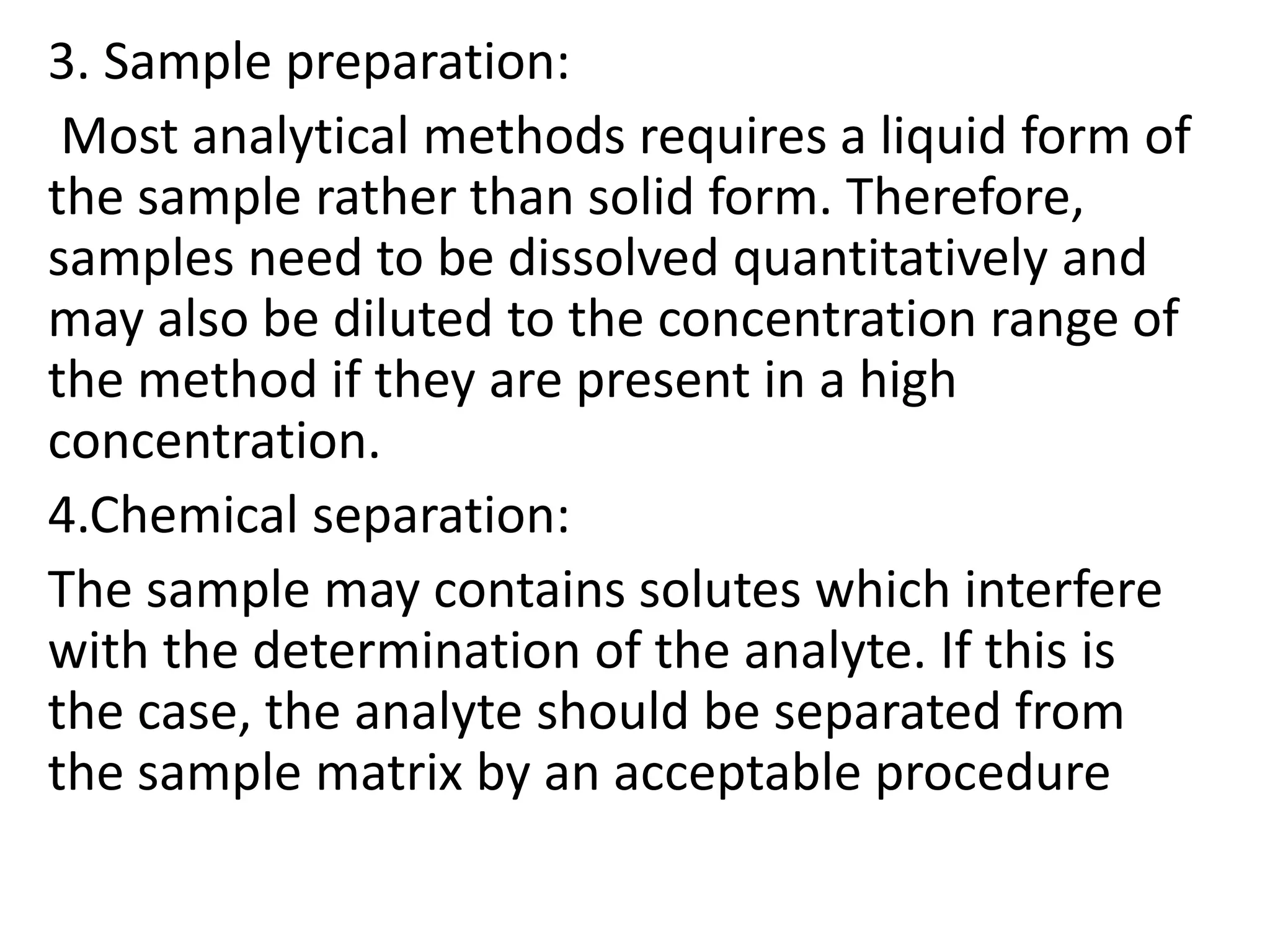
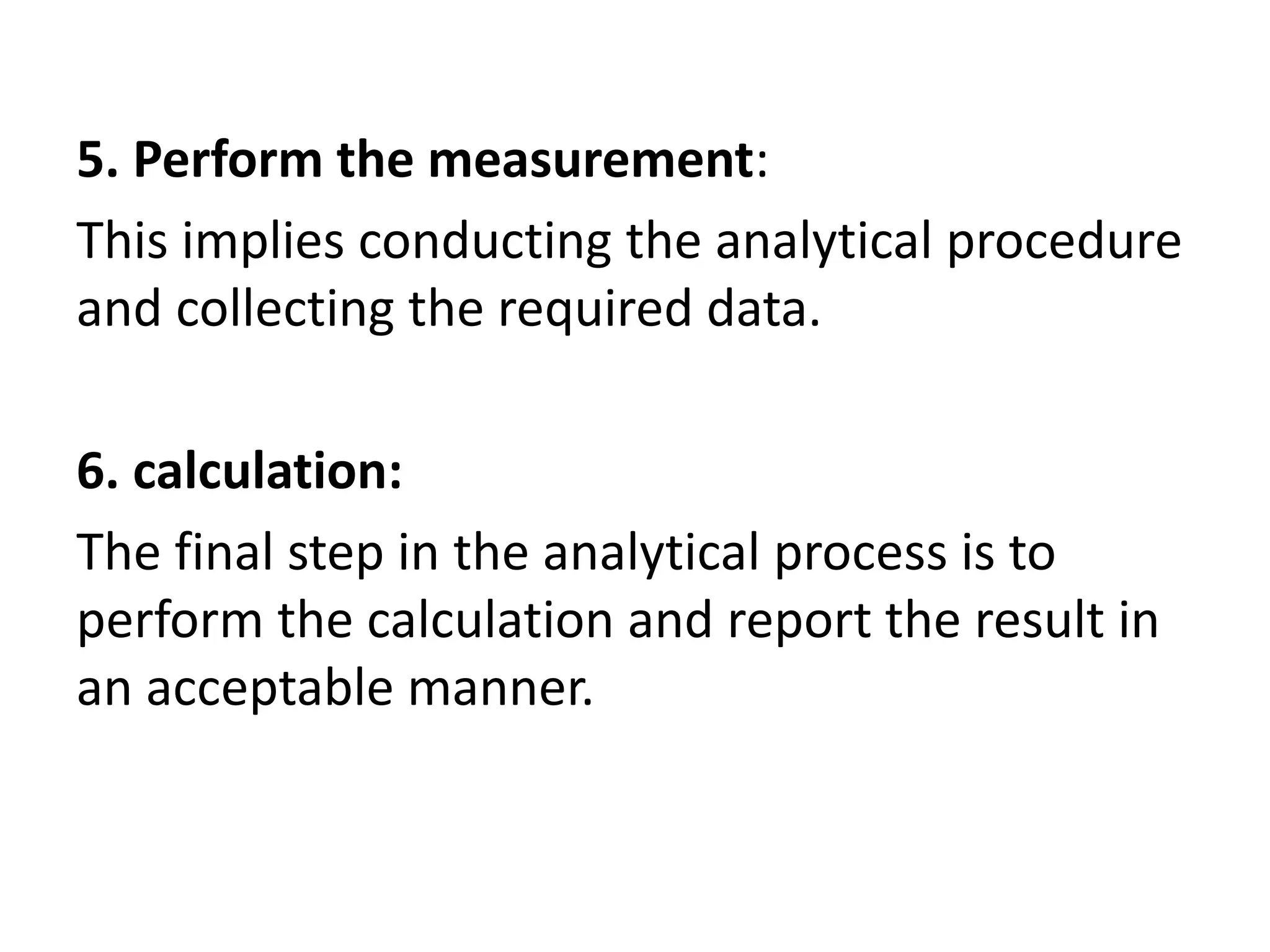
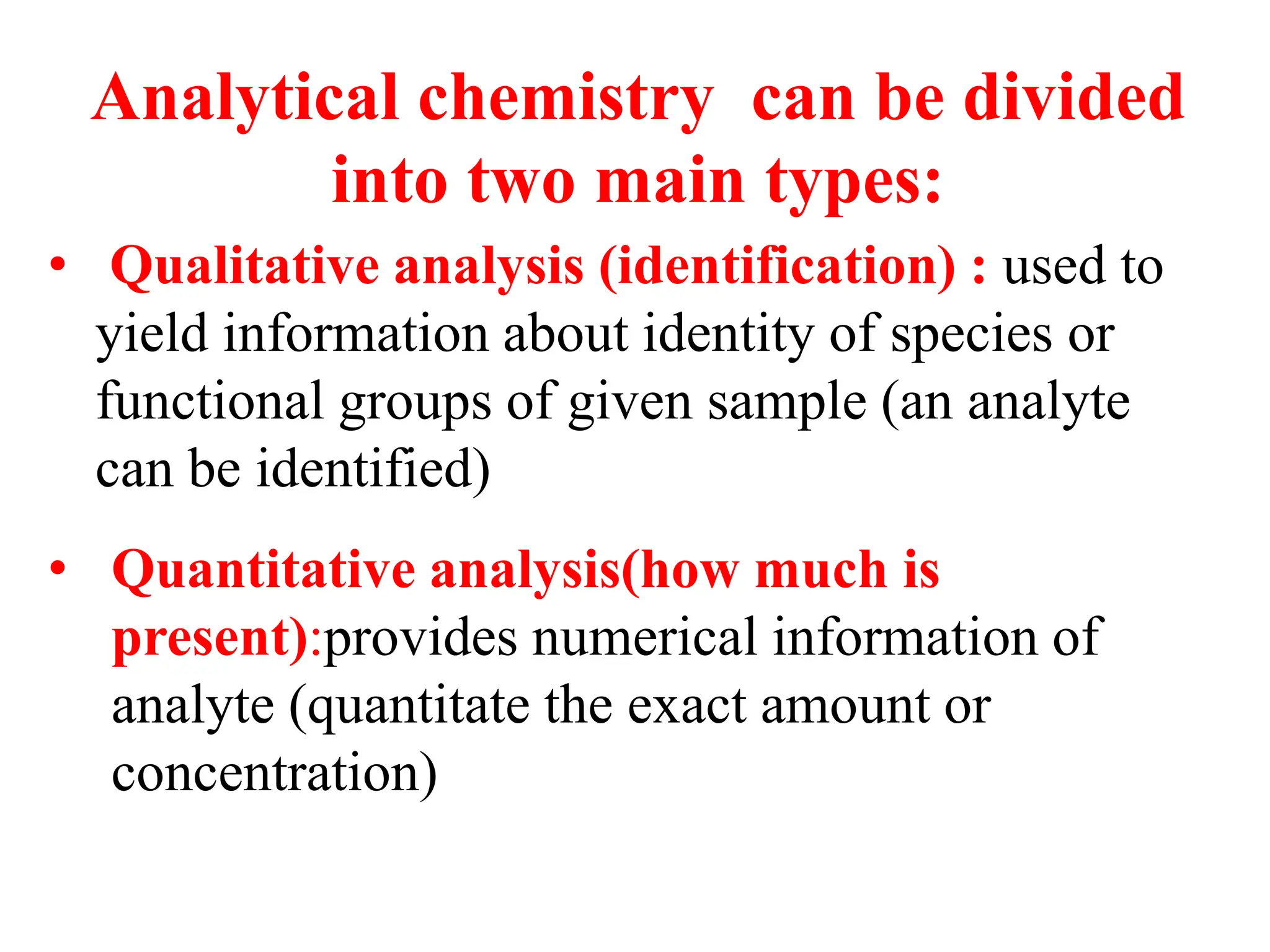
The document provides an introduction to analytical chemistry, defining its scope, classification, and the importance of gravimetric analysis. It details various steps involved in analytical analysis, including problem definition, sample preparation, chemical separation, measurement, and calculation, while outlining types of analysis: qualitative and quantitative. The text also highlights methods used in quantitative analysis, such as gravimetric and volumetric methods, and emphasizes the significance of analytical chemistry in areas like drug manufacturing, environmental monitoring, and medical diagnostics.