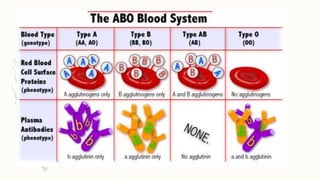
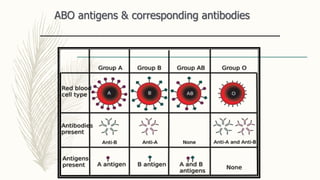
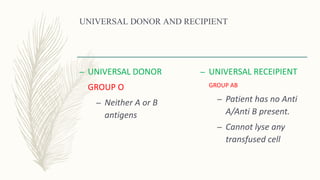
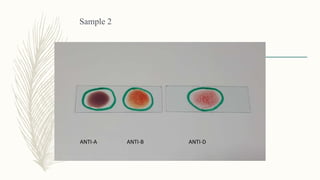
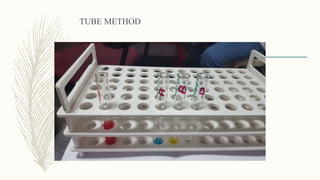
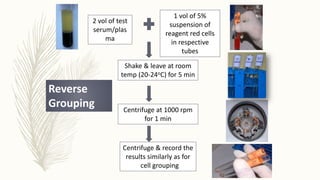
This document discusses the importance of blood grouping and Landsteiner's law of blood grouping. It describes the ABO blood group system and the universal donor and recipient groups. The key blood grouping techniques of slide method and tube method are outlined. The slide method is a preliminary test while the tube method is the gold standard. The document explains the process of cell grouping (forward grouping) and serum grouping (reverse grouping) using the tube method. It also discusses grading of agglutination results and the importance of cross-matching blood prior to transfusion to avoid mismatched reactions.