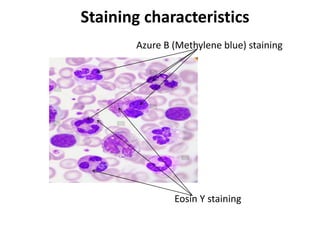
The document provides an in-depth overview of Romanowsky stains, including their definition, principles, types, preparation methods, and uses in hematology and cytology. It discusses the staining characteristics, specific preparation techniques for various stains (such as Giemsa, May-Grünwald, Wright's, and Leishman), and outlines quality control procedures to ensure reliable results. Overall, it serves as a comprehensive guide for the application of Romanowsky stains in laboratory settings.