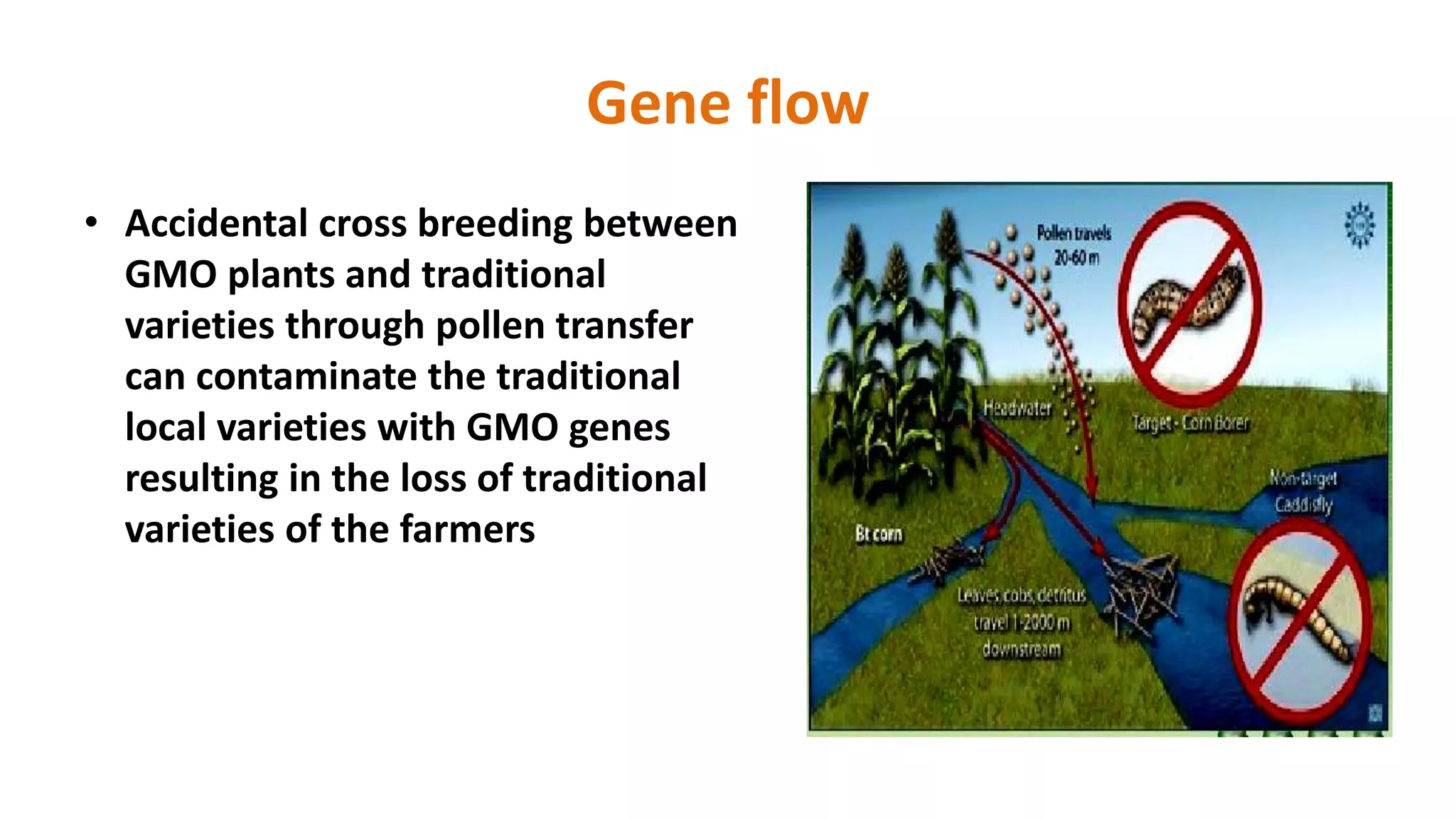
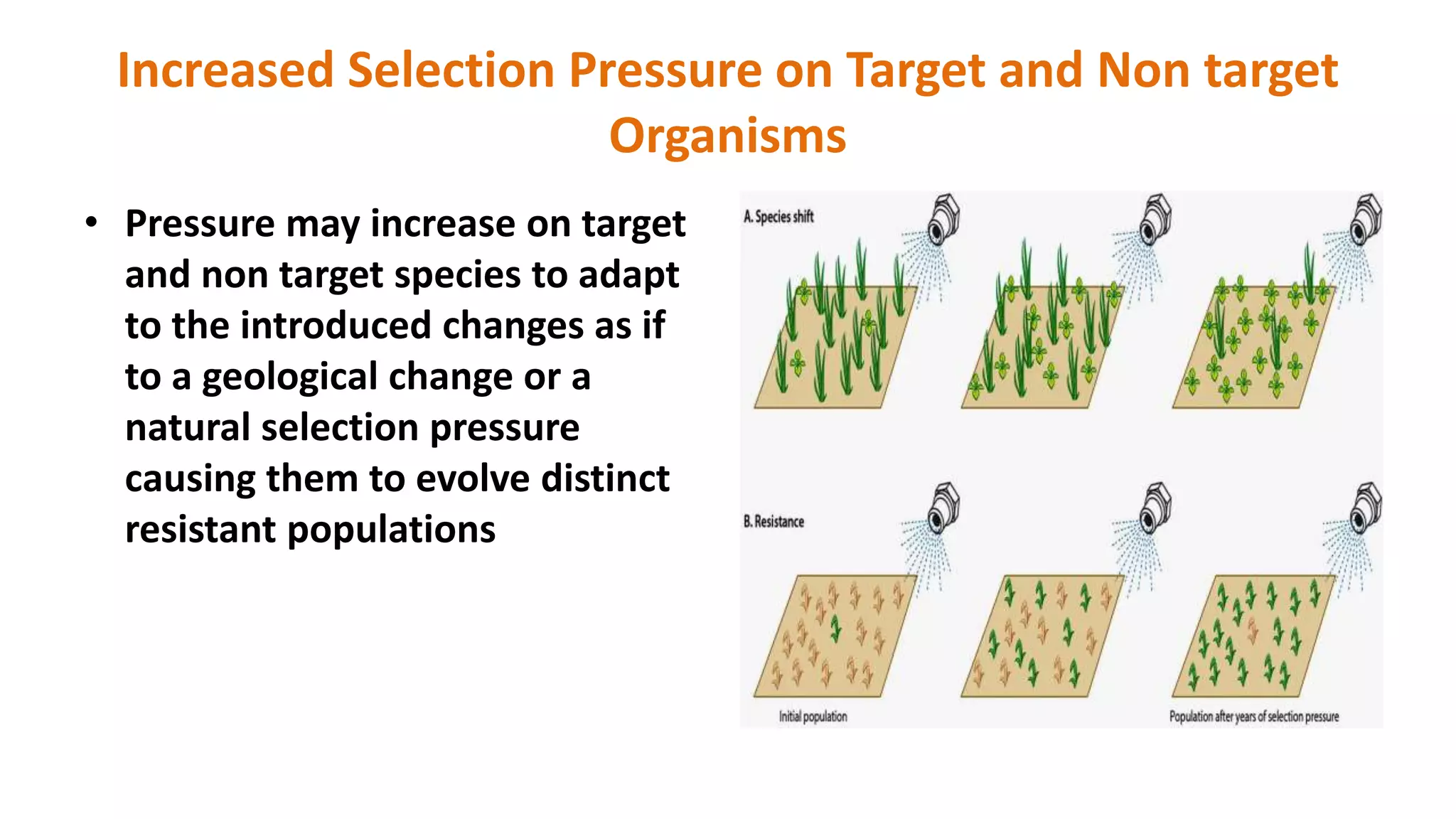
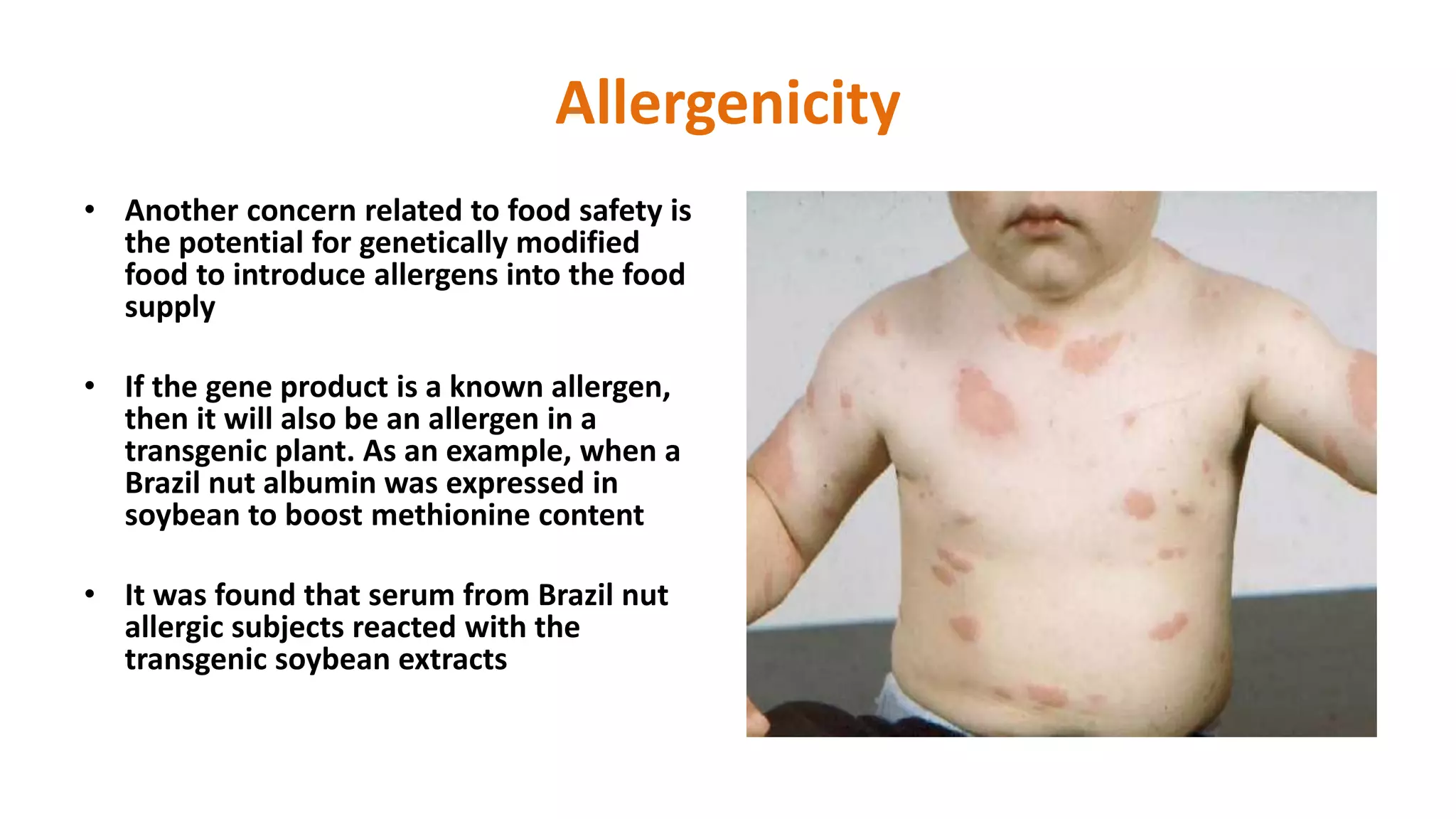
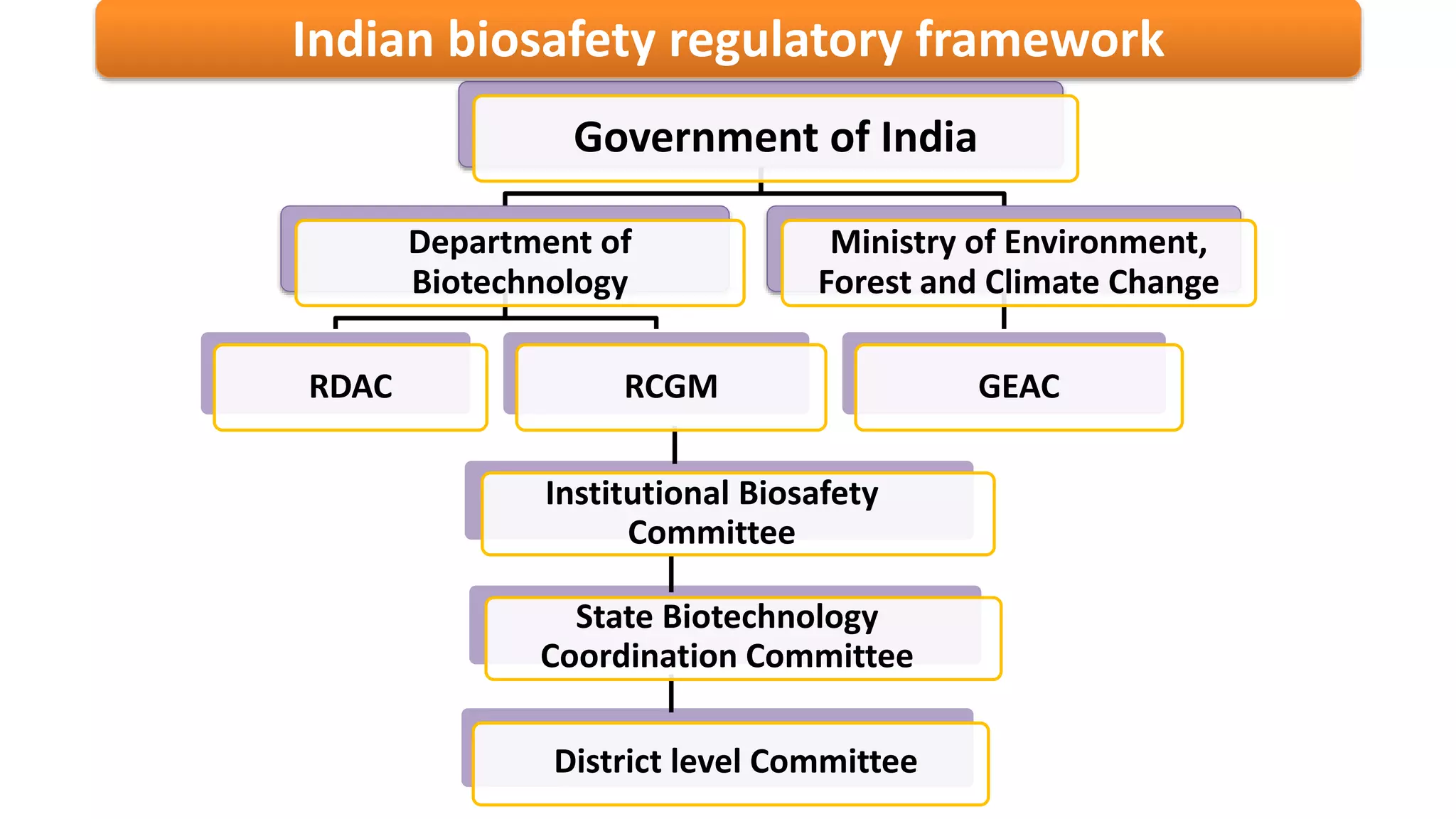
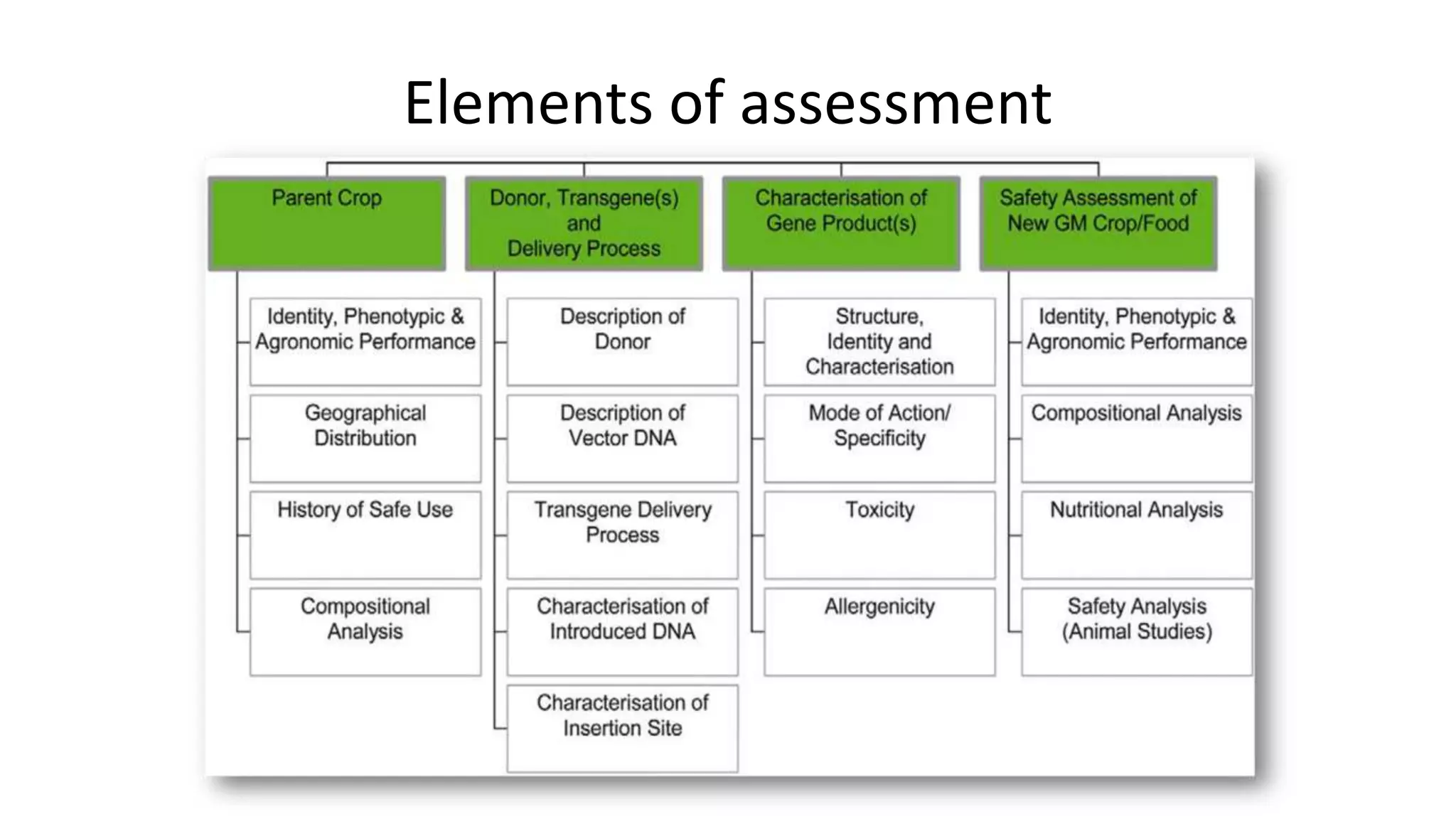
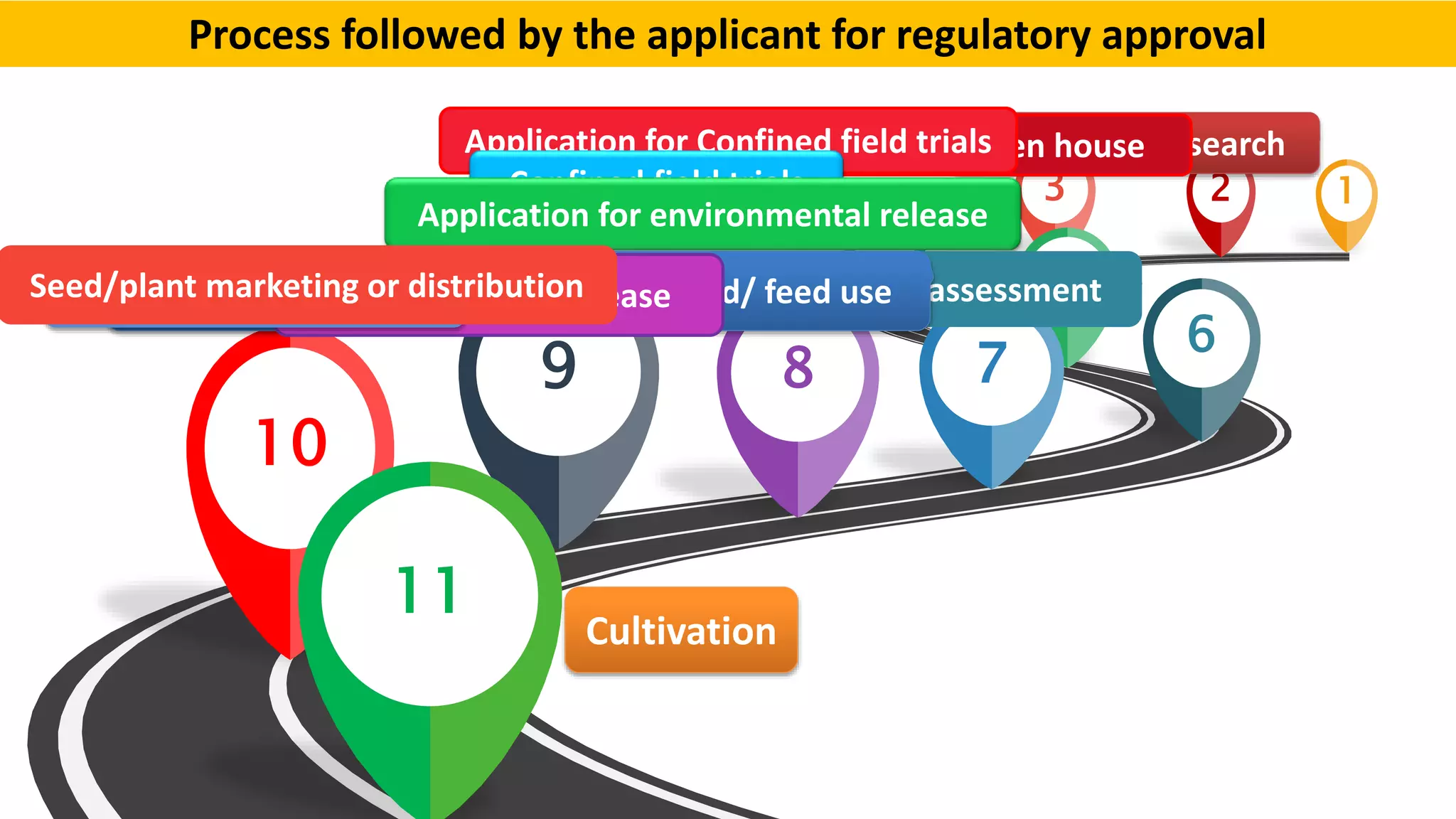
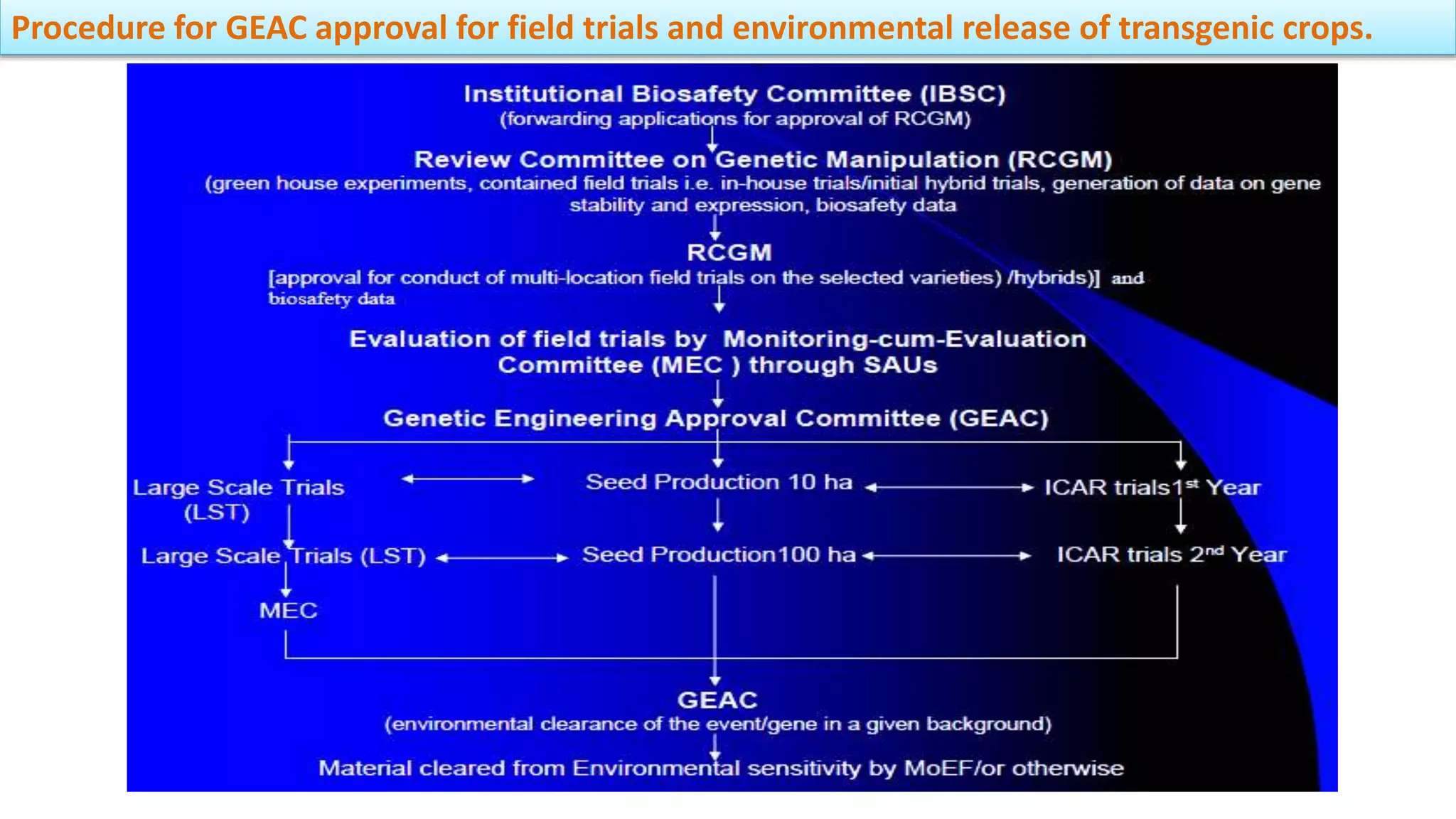
The document discusses issues related to biosafety and registration of transgenic agricultural organisms. It outlines three main biosafety concerns: environmental safety, food safety for human and animal health, and risk management. Some potential environmental risks discussed include effects on non-target organisms, development of insect resistance, gene flow, increased weediness, loss of biodiversity, changes in soil ecology, and genetic contamination. Food safety concerns include toxicity, allergenicity, and unintended effects. The document also describes India's biosafety regulatory framework and approval process for transgenic crops, which involves biosafety assessment and approval from multiple government committees and agencies before crops can be cultivated and marketed.