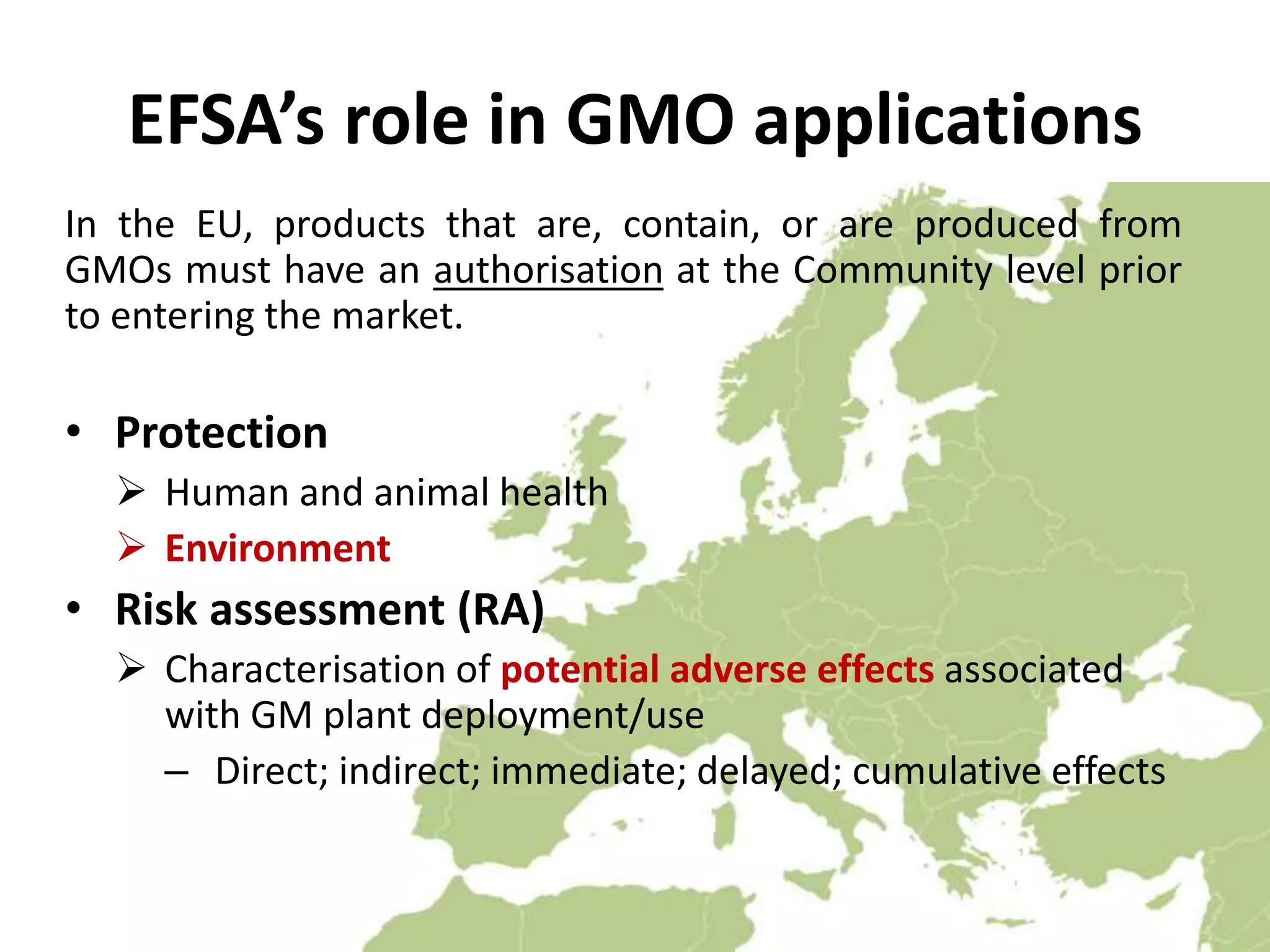
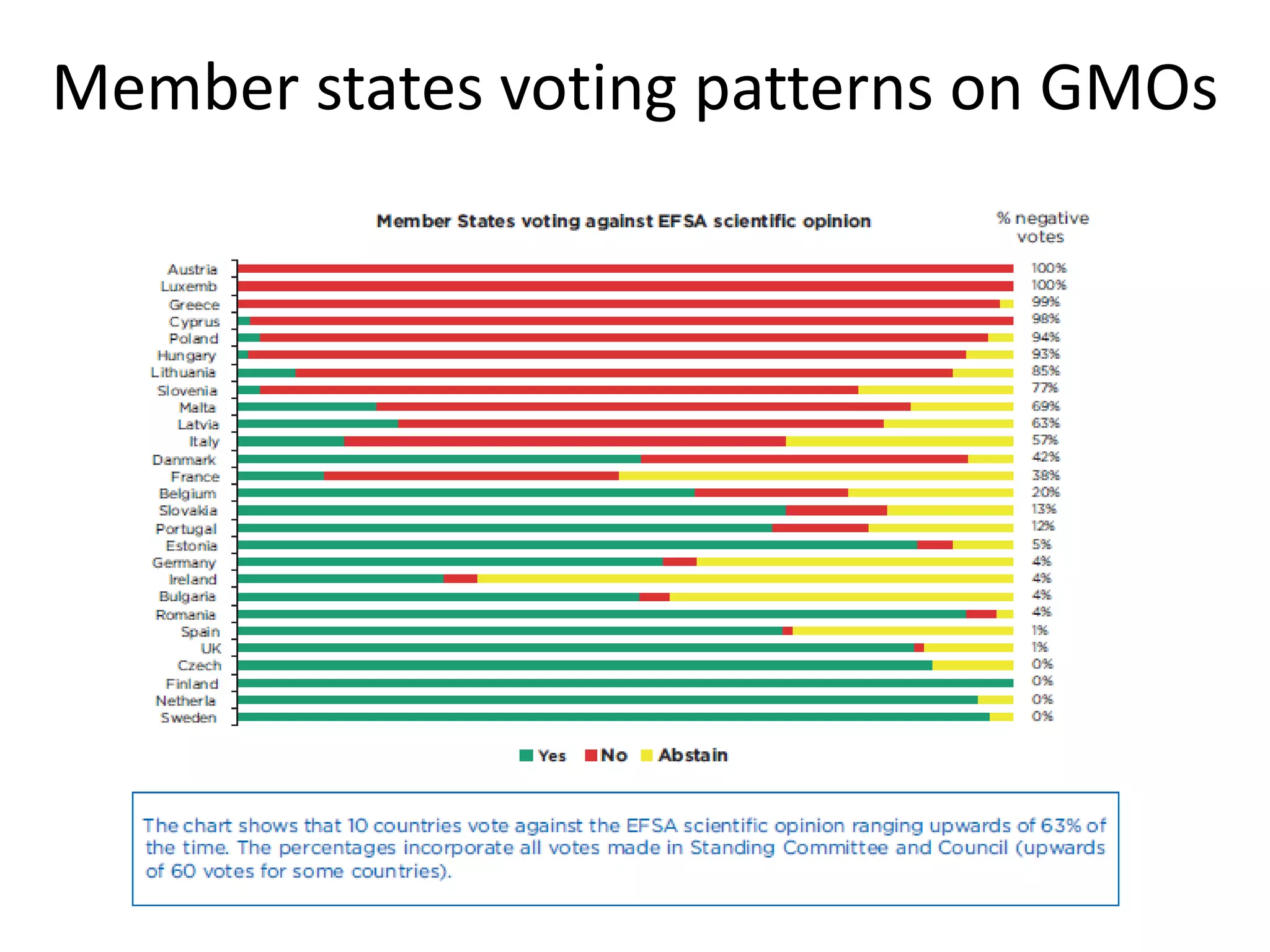
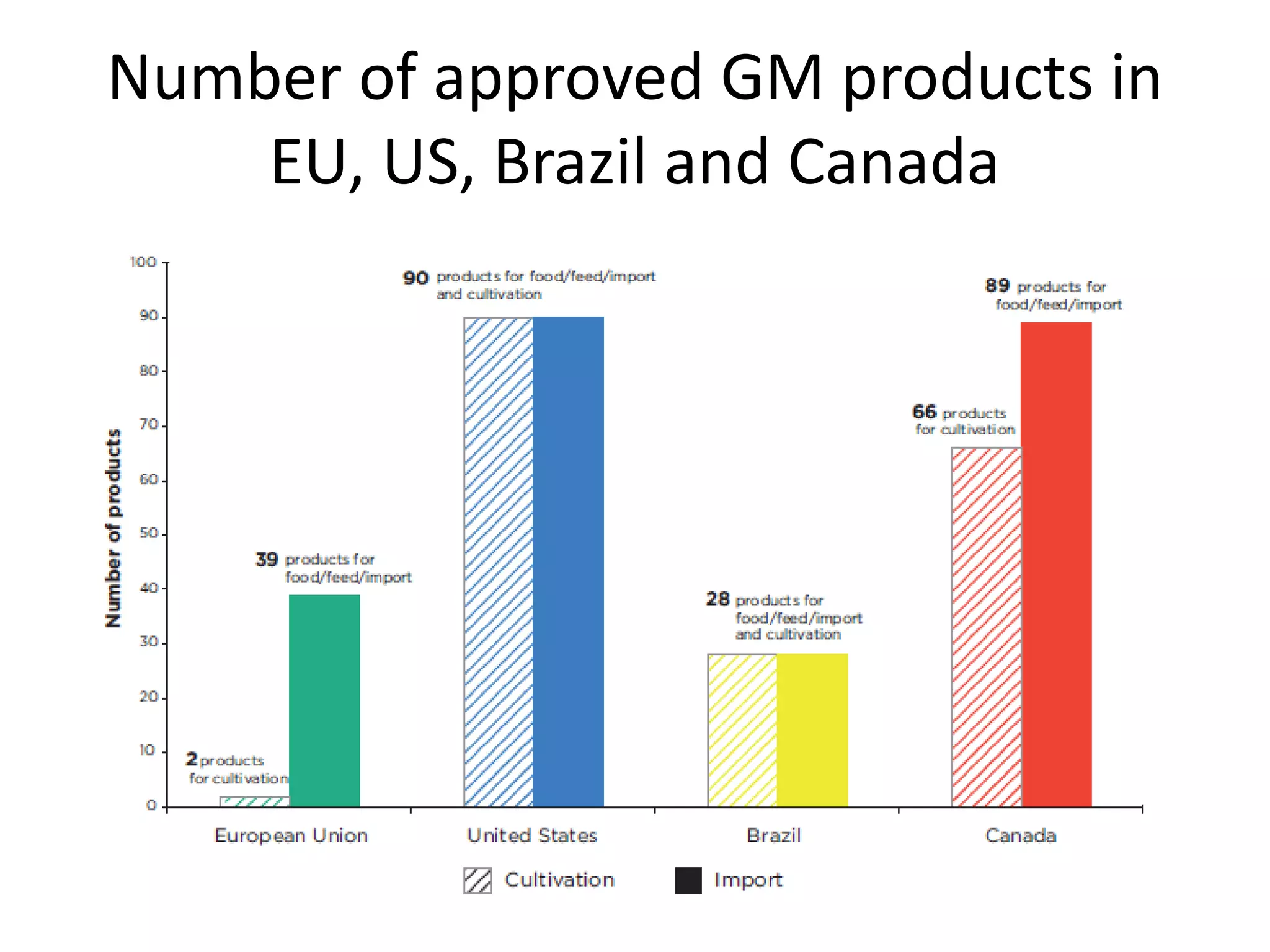
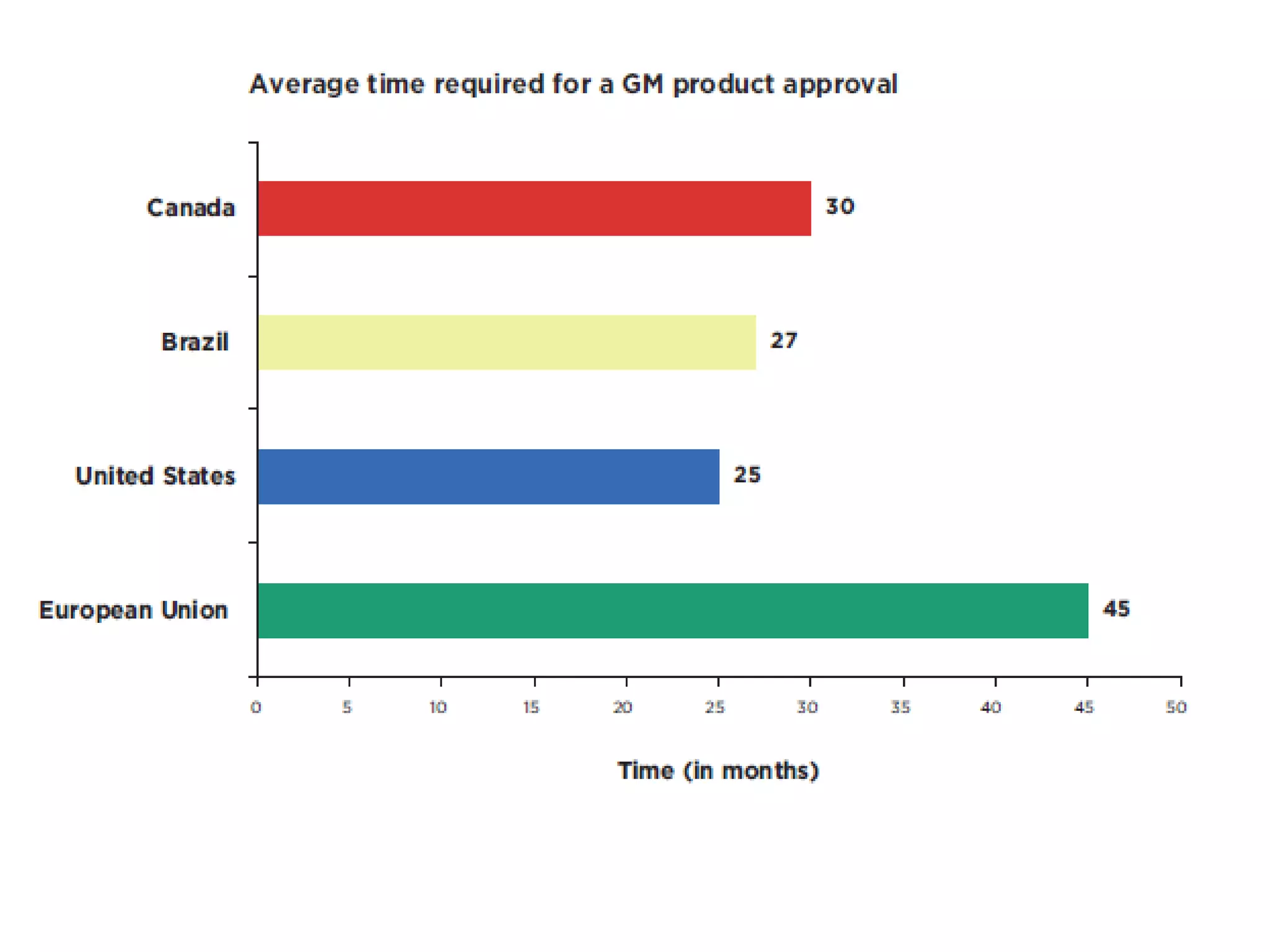
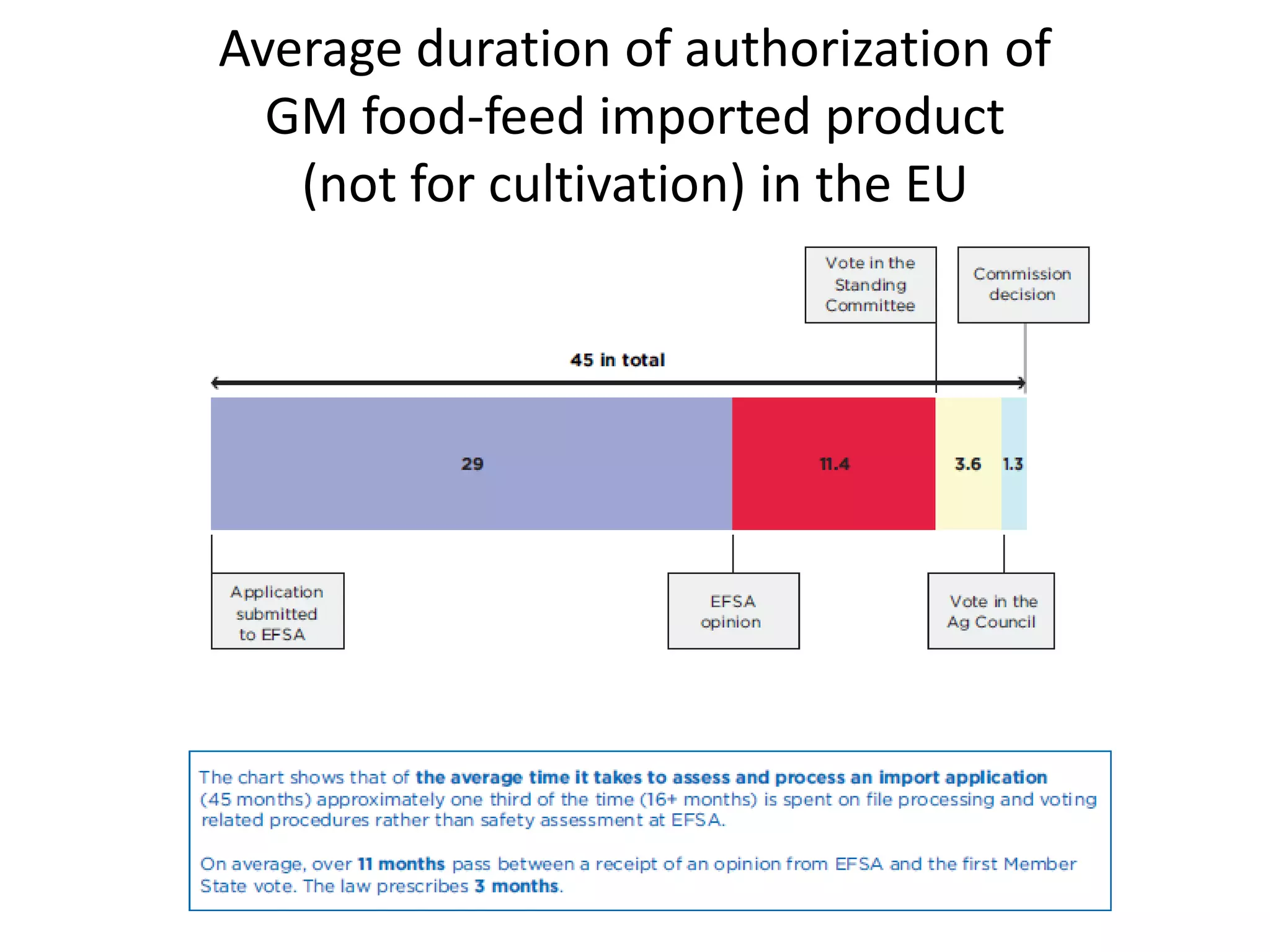
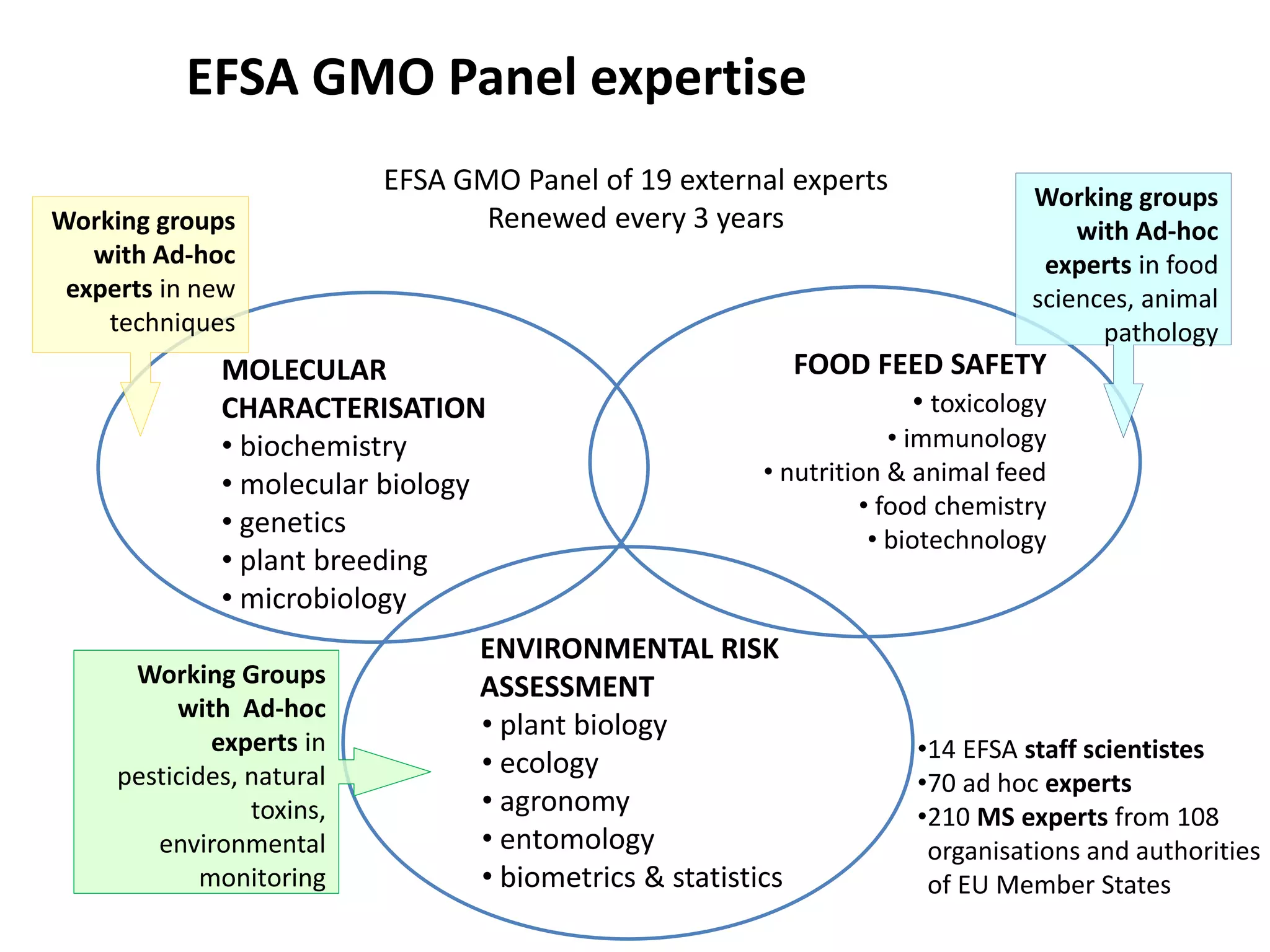
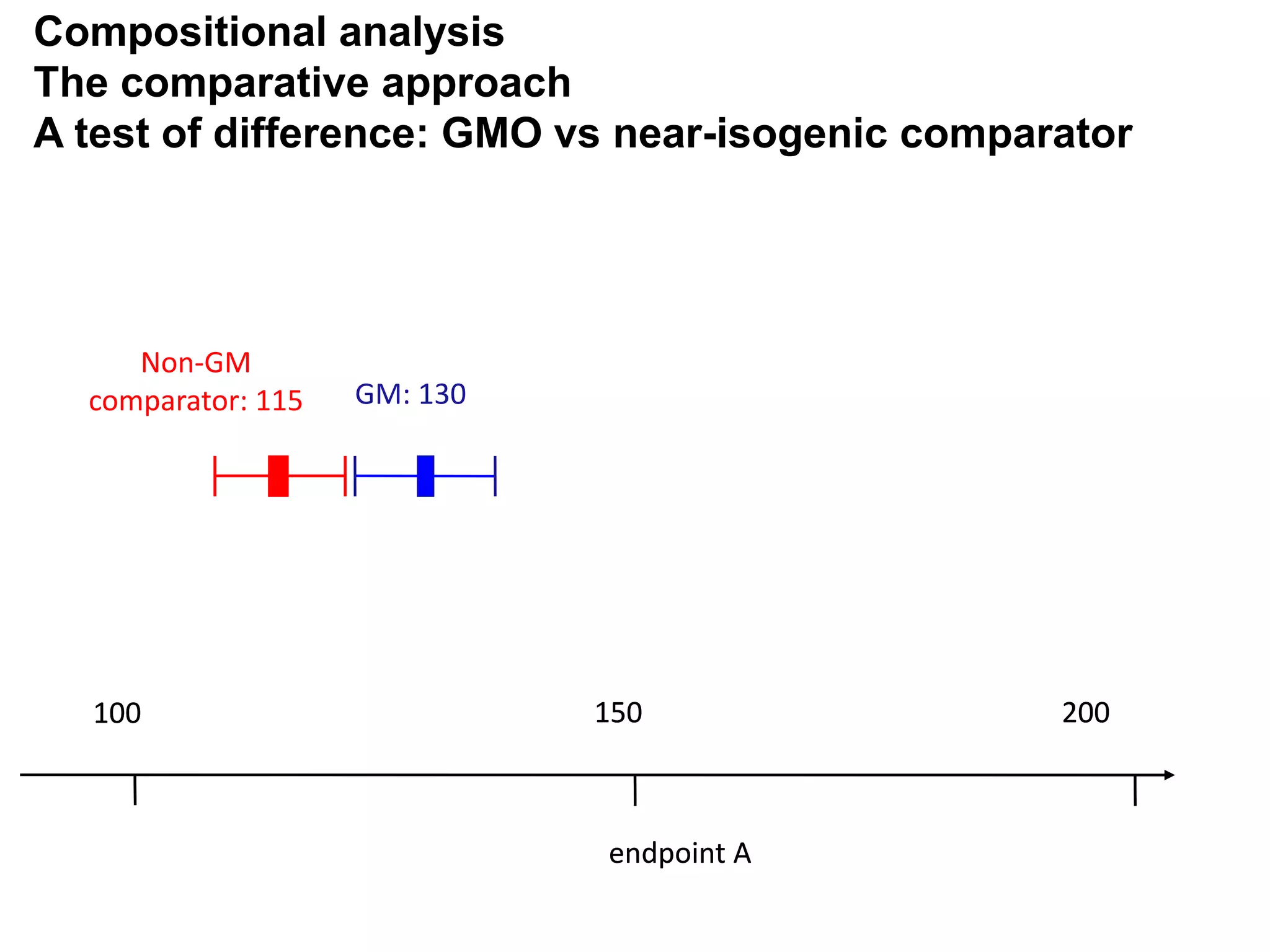
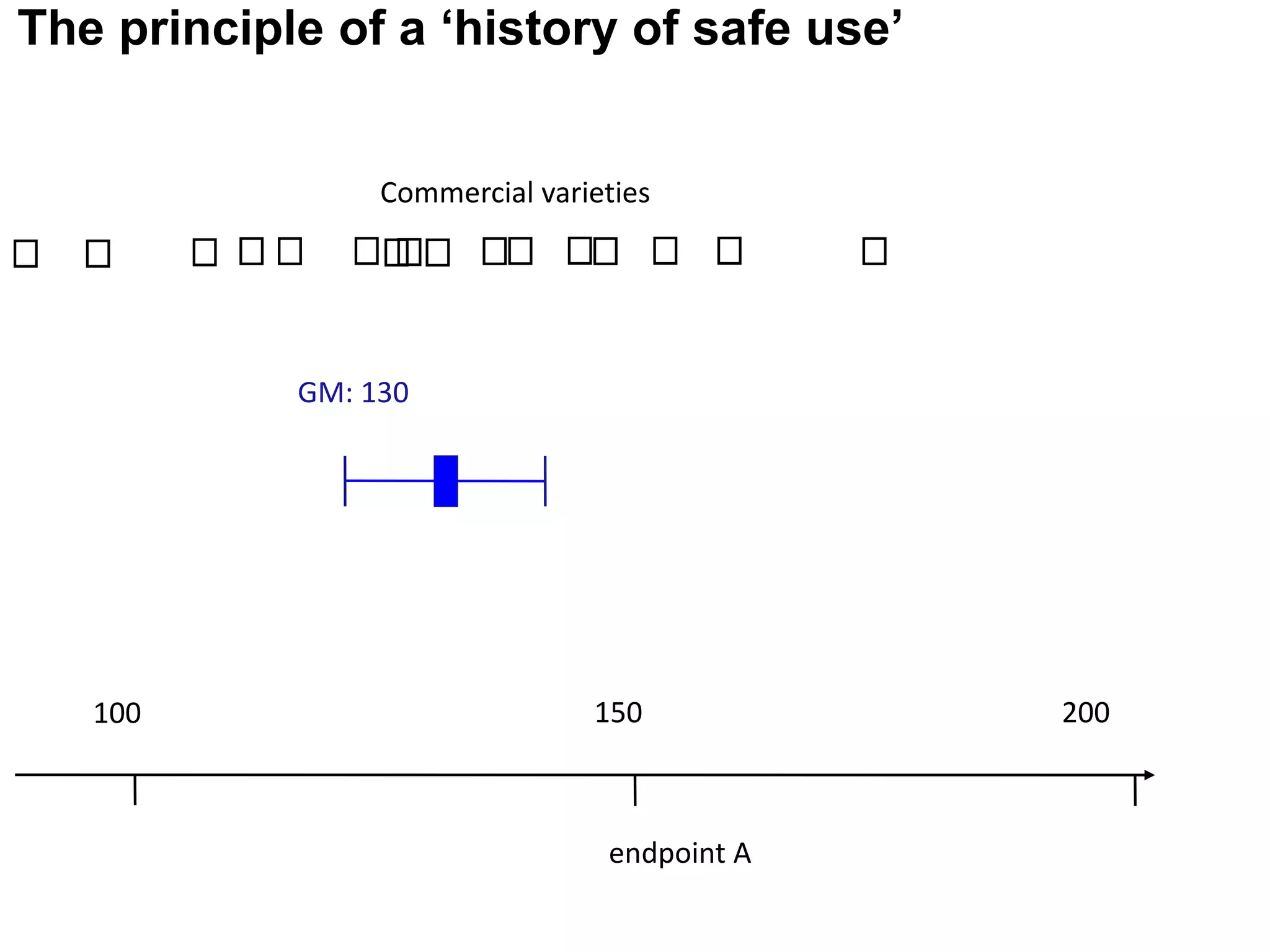
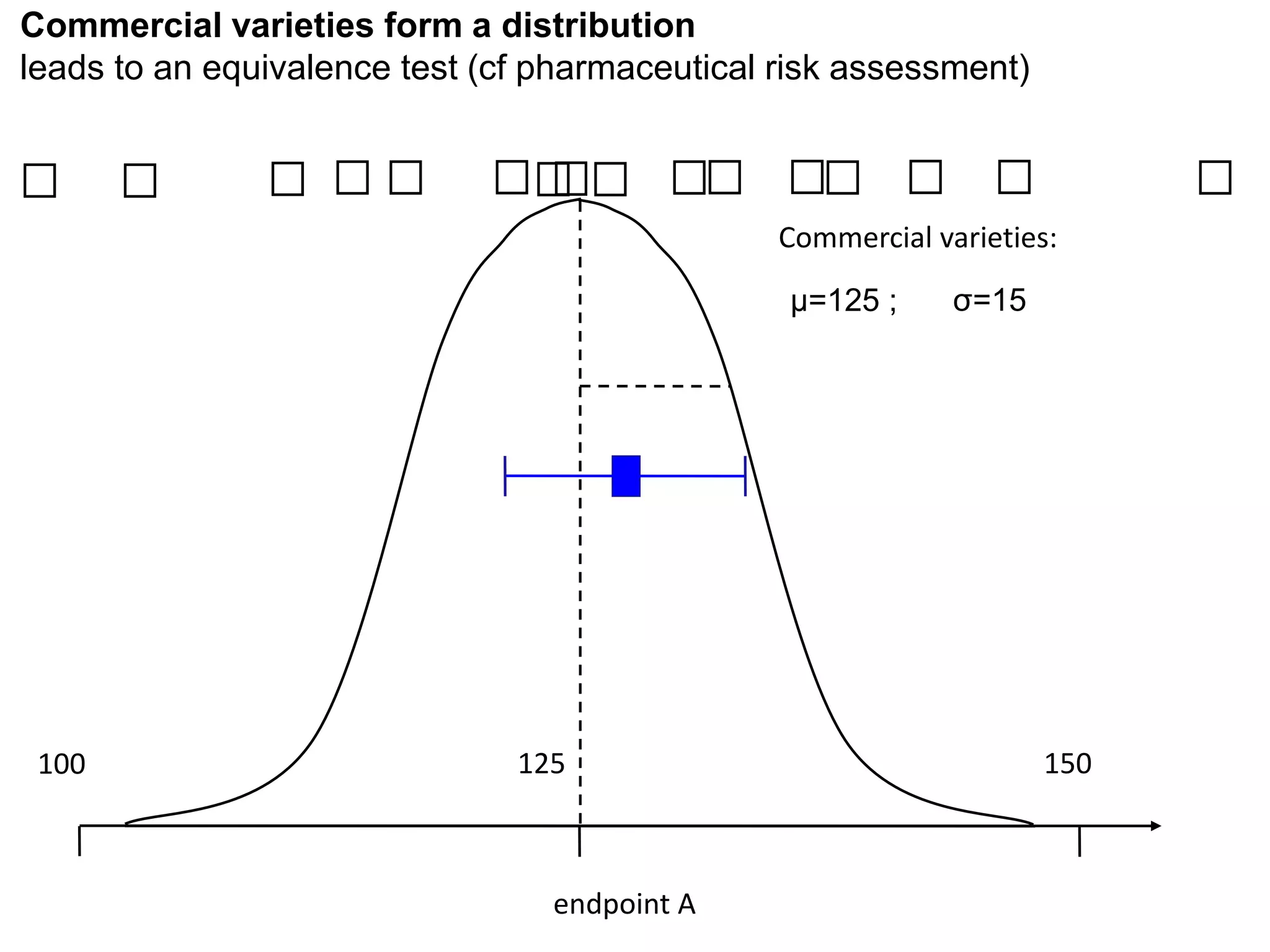
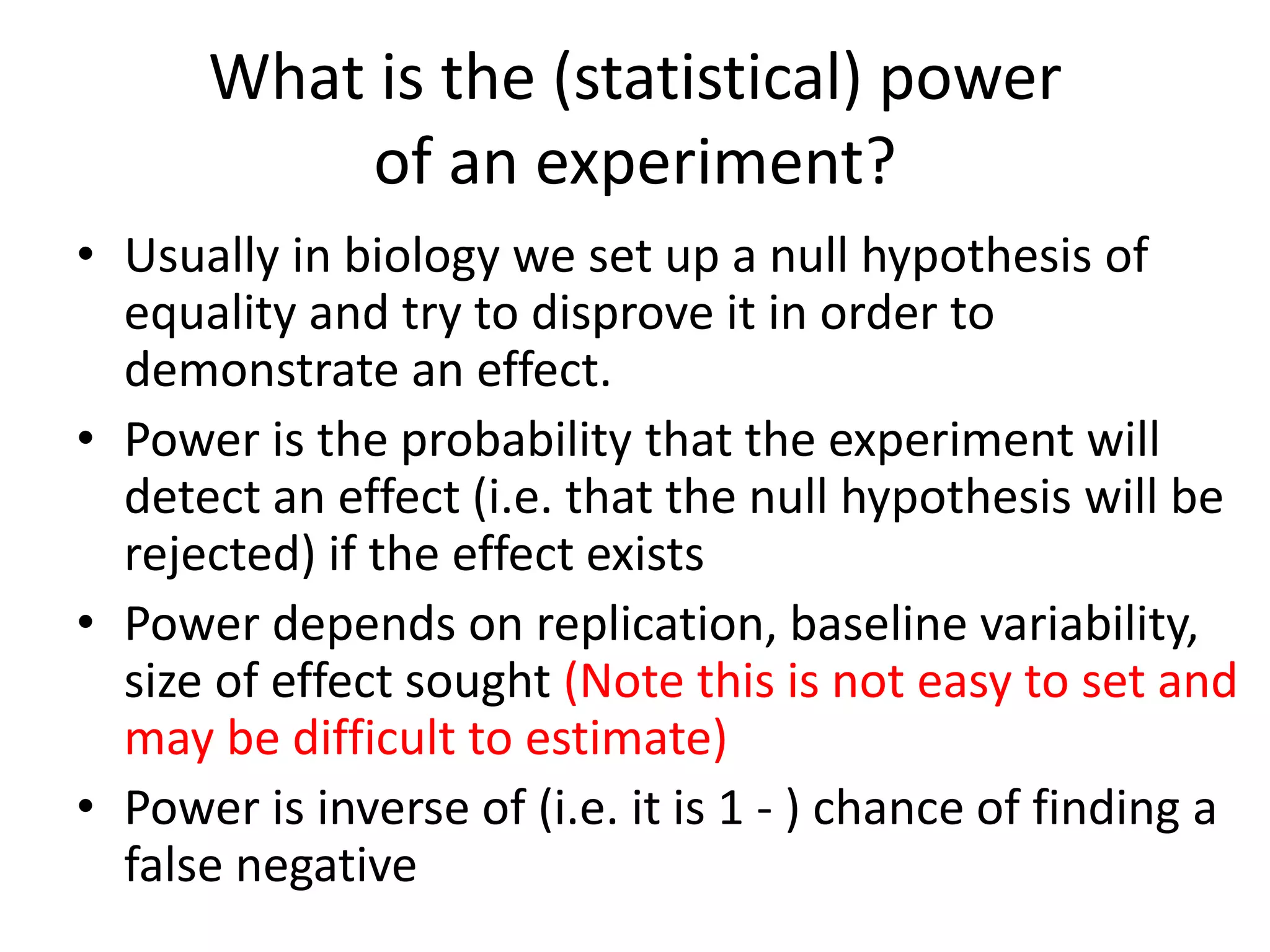
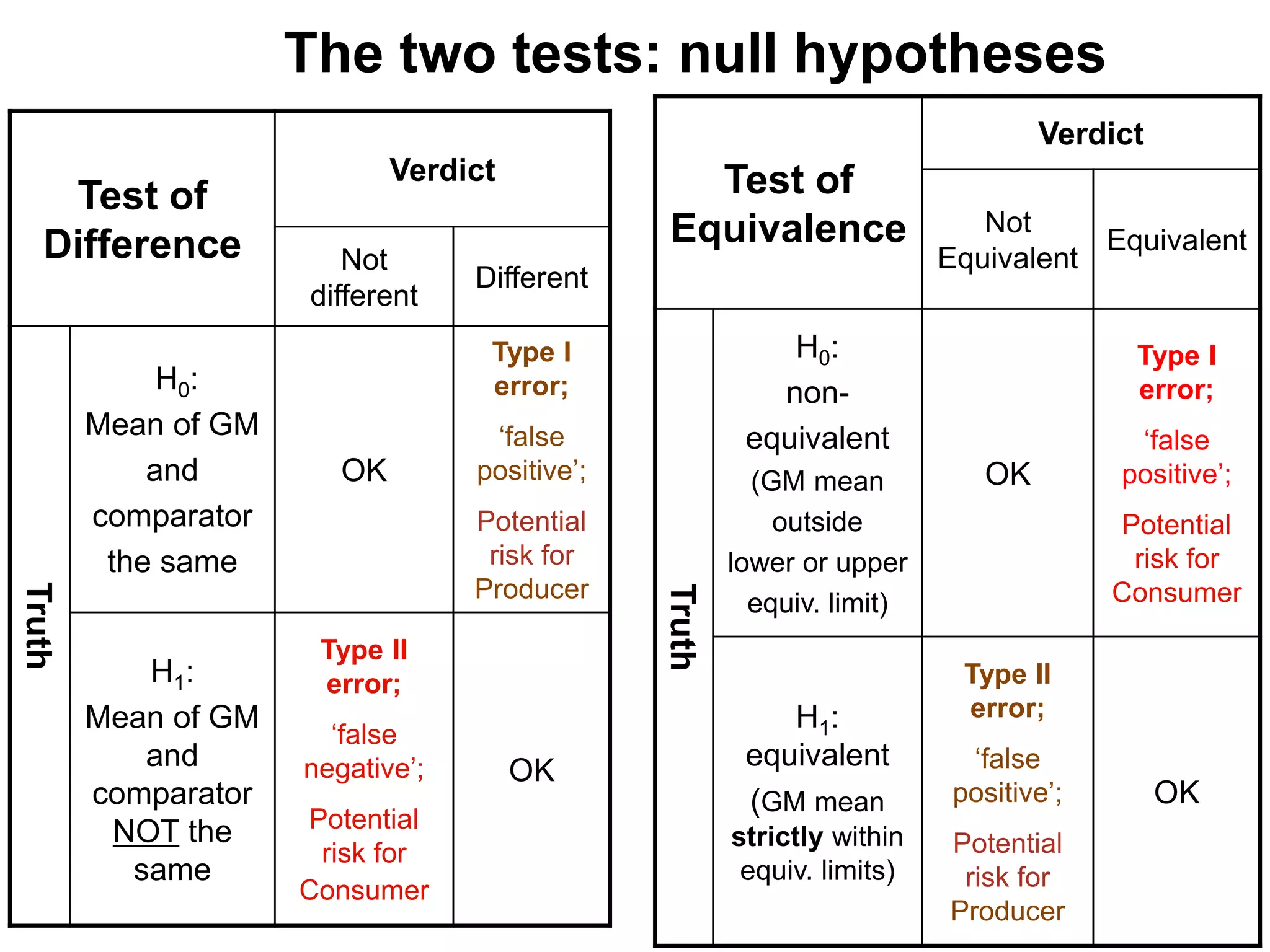
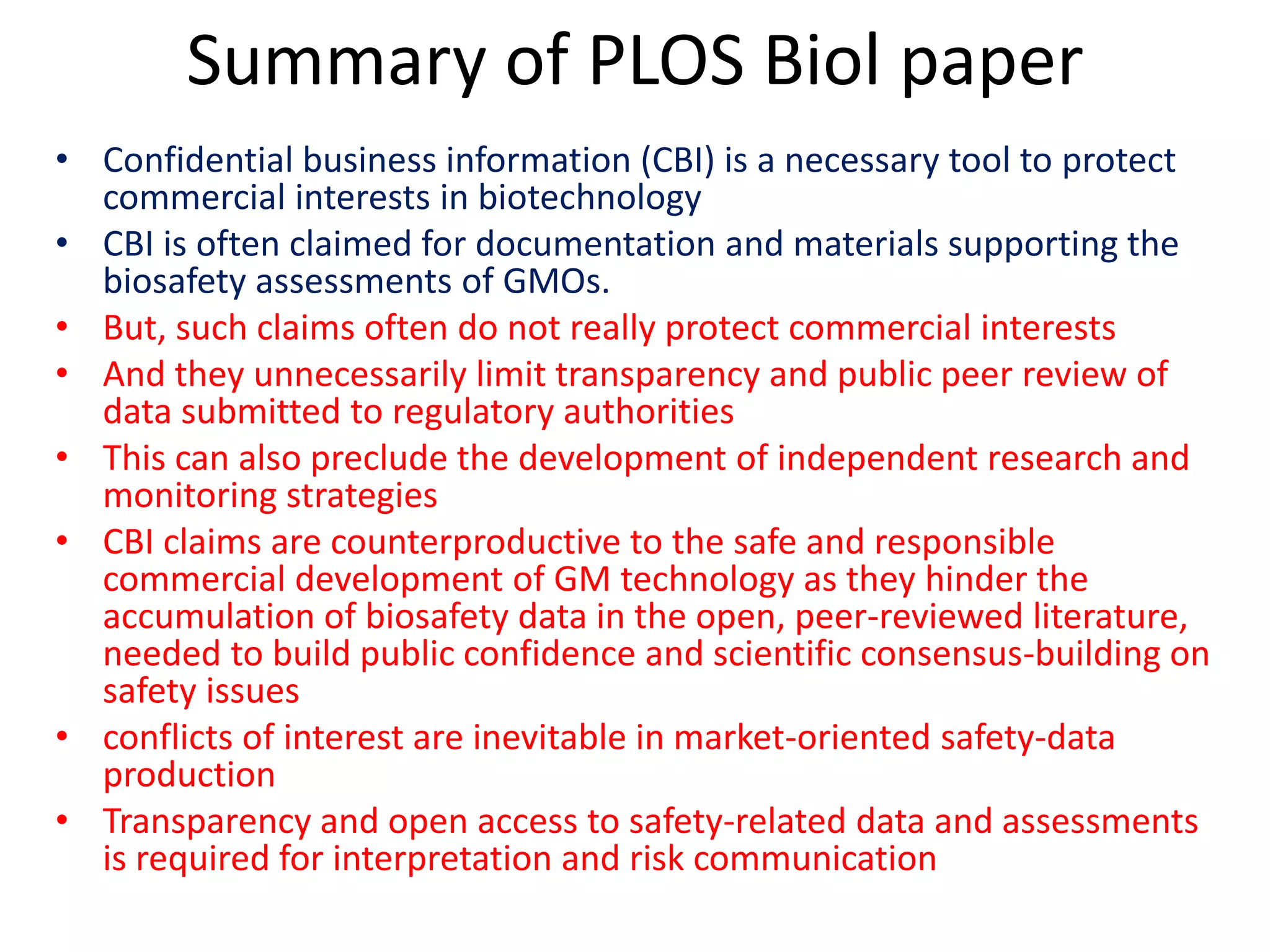
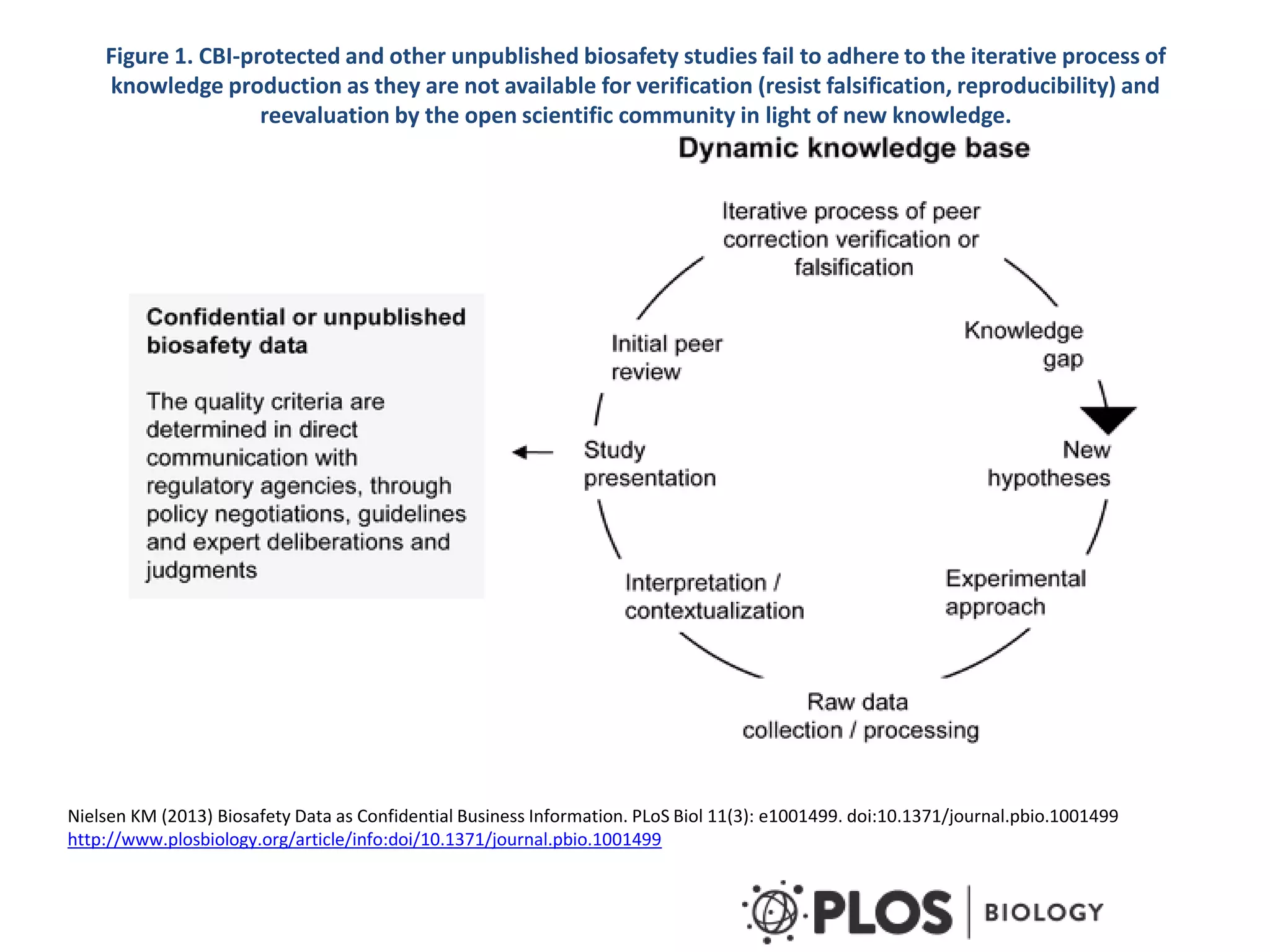
The document discusses the assessment and regulation of genetically modified organisms (GMOs) in Europe, outlining the roles of the European Food Safety Authority (EFSA) and the regulatory framework established by directives 2001/18 and 1829/2003. It highlights the complexities and risks associated with GMOs, including socio-economic and ecological effects, and emphasizes the need for transparency in safety assessments to build public confidence. Additionally, it addresses future challenges posed by emerging biotechnologies and the potential risks associated with new traits and genetically modified animals.