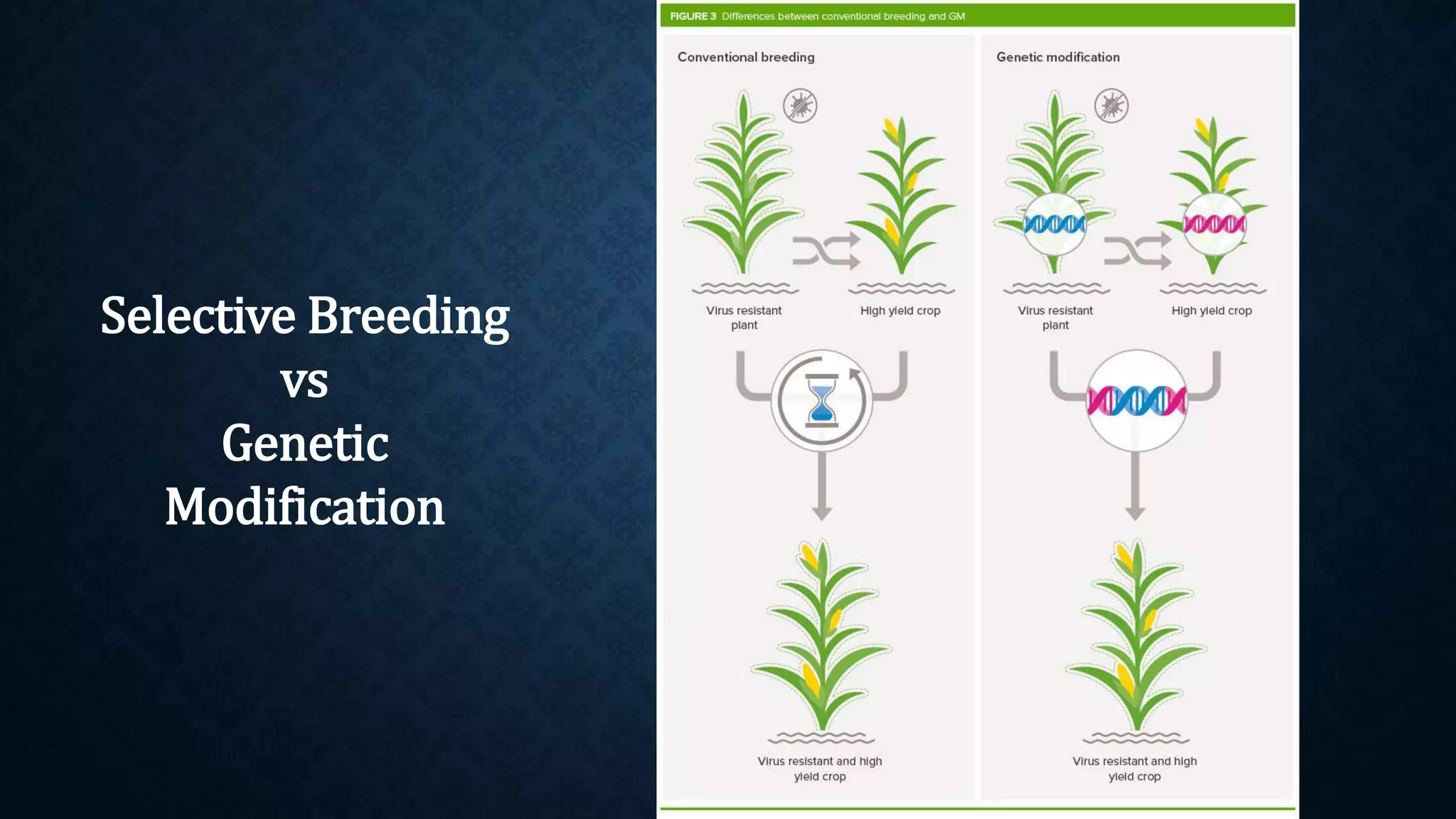
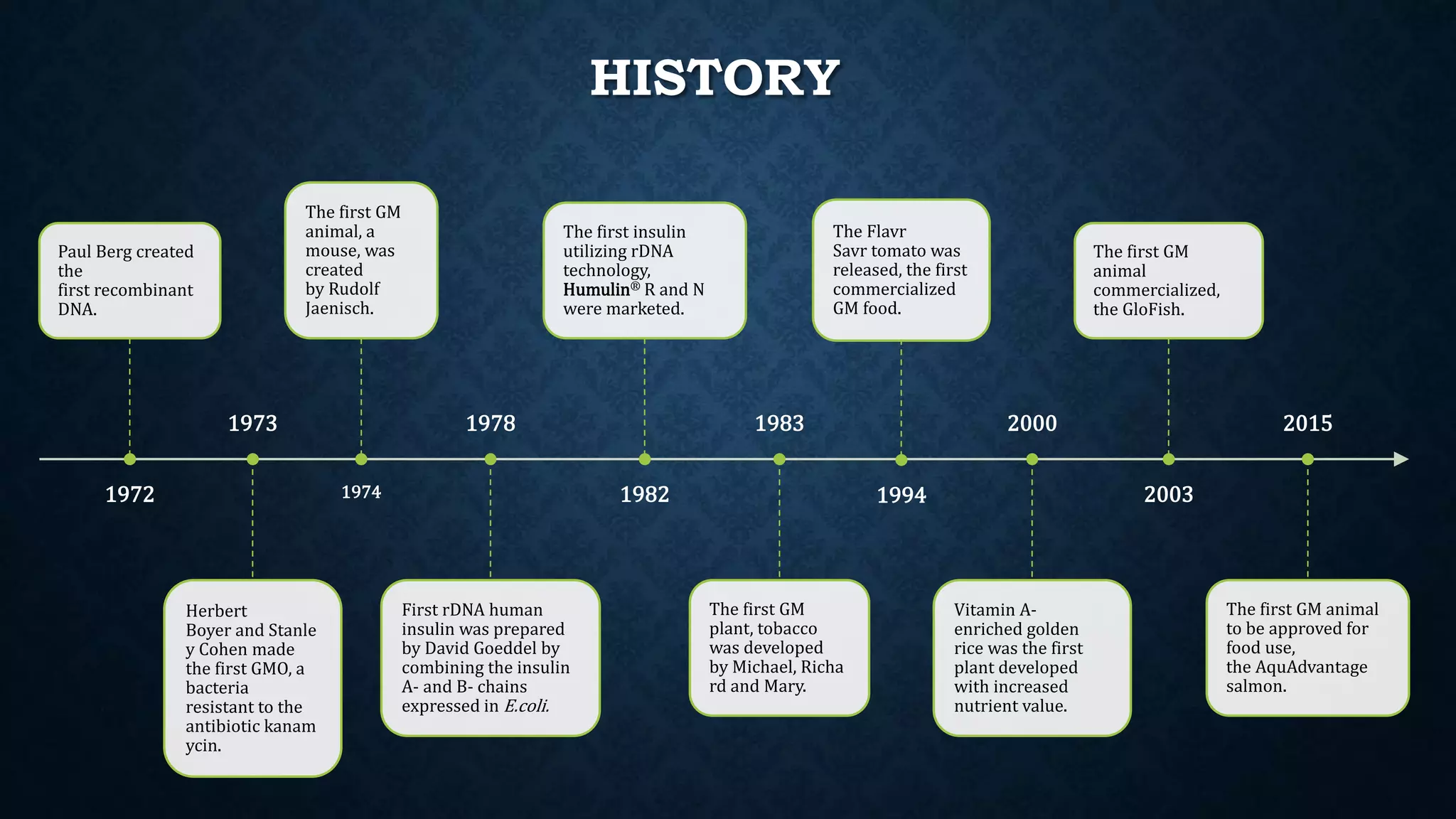
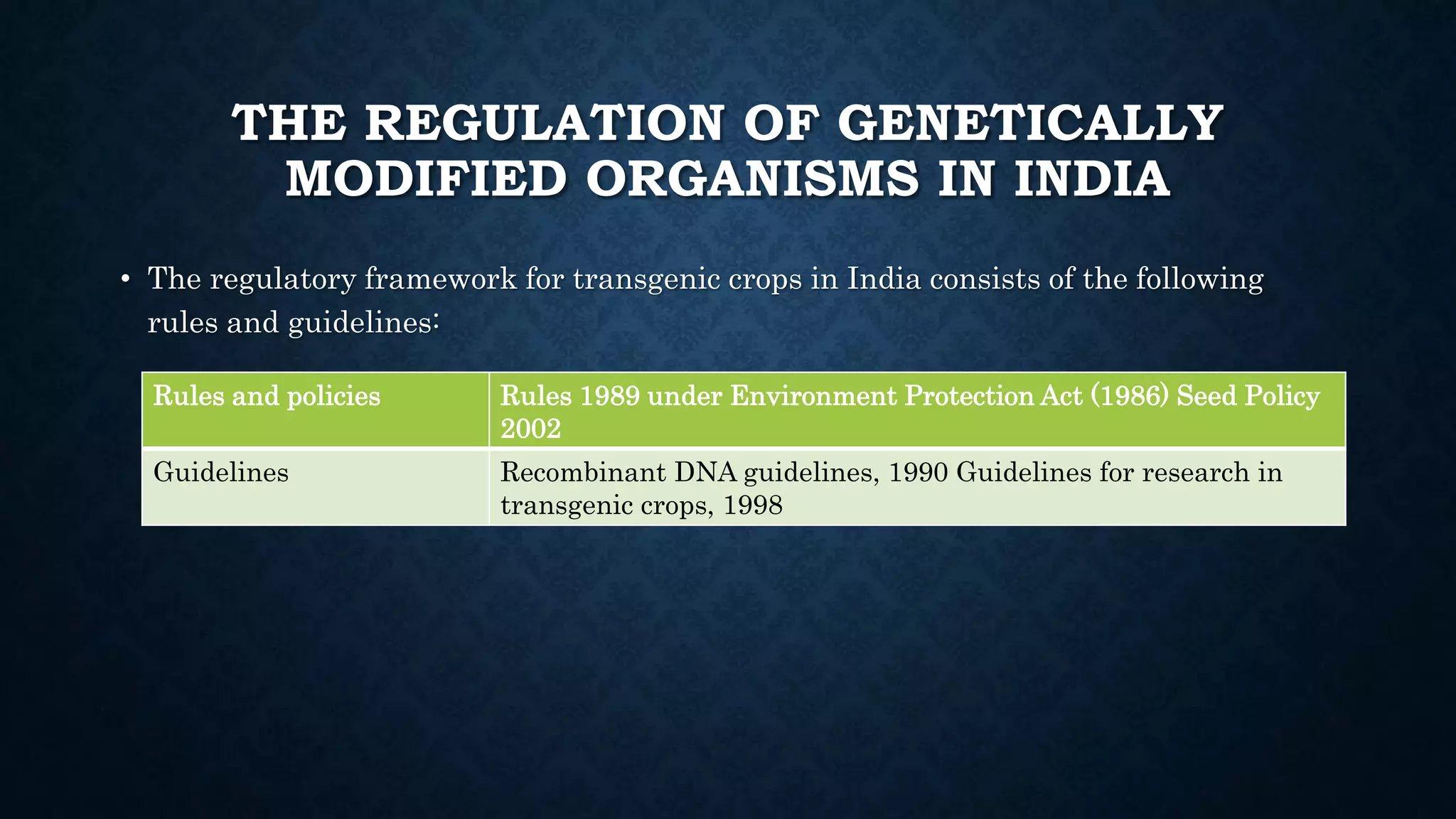
The document discusses genetically modified organisms (GMOs), their history, development, and associated public concerns regarding biosafety, environmental impact, and health risks. It outlines the steps involved in creating GMOs and highlights the regulatory framework governing their approval in India, emphasizing the extensive testing required before commercialization. Issues raised include potential allergenicity, unintended environmental effects, and the overarching dominance of biotech corporations over farmers through patented genetic technologies.