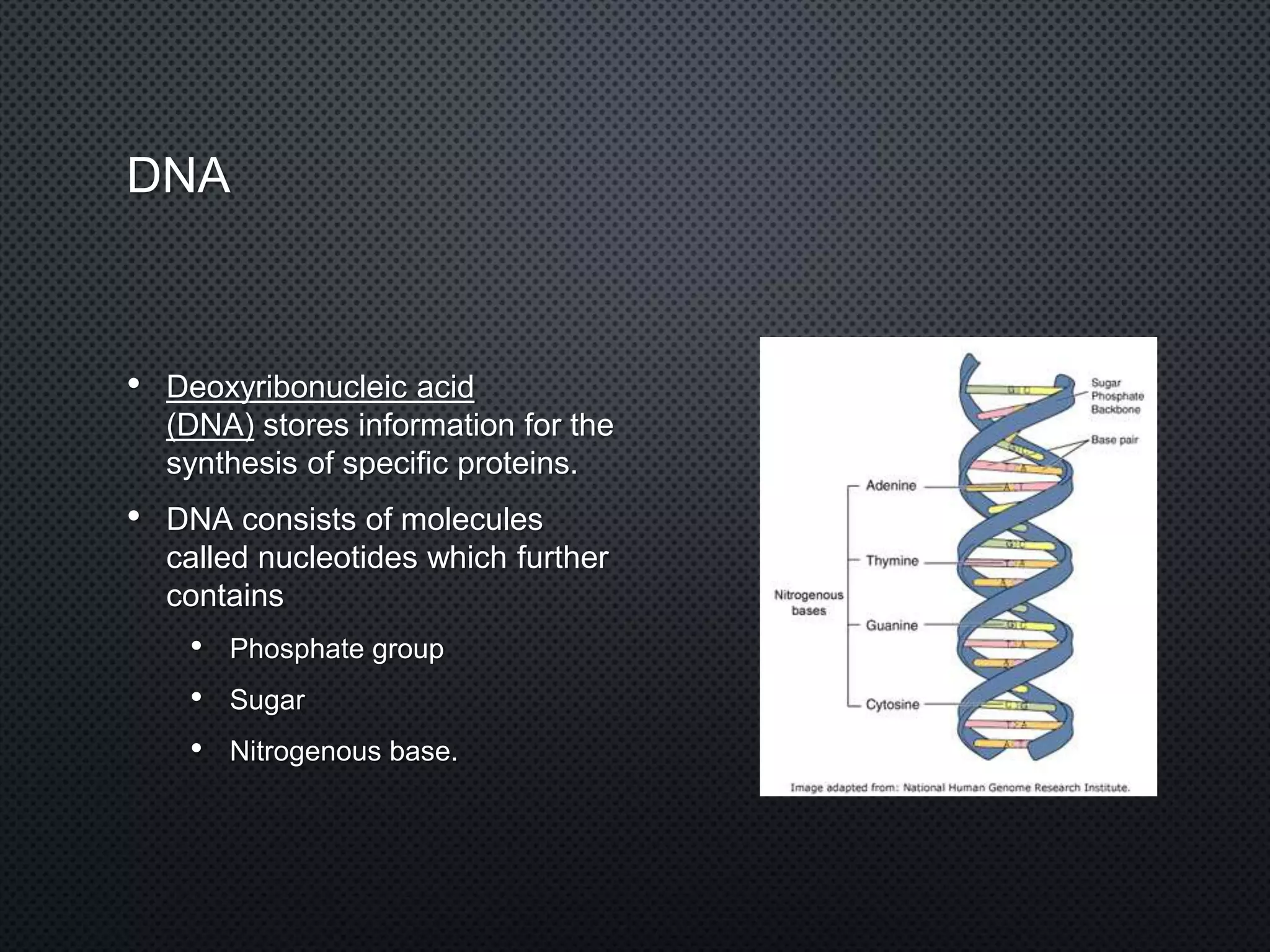
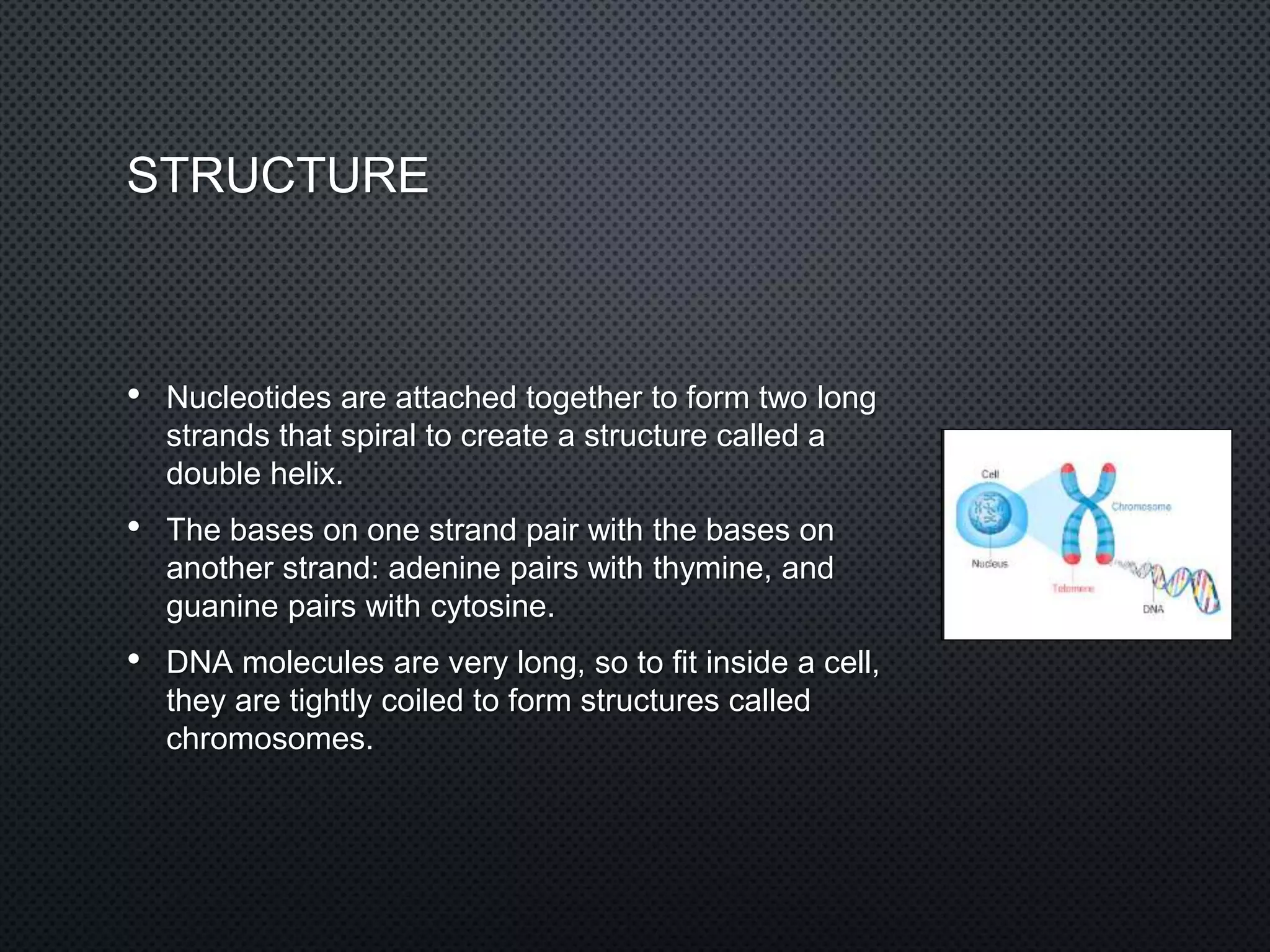
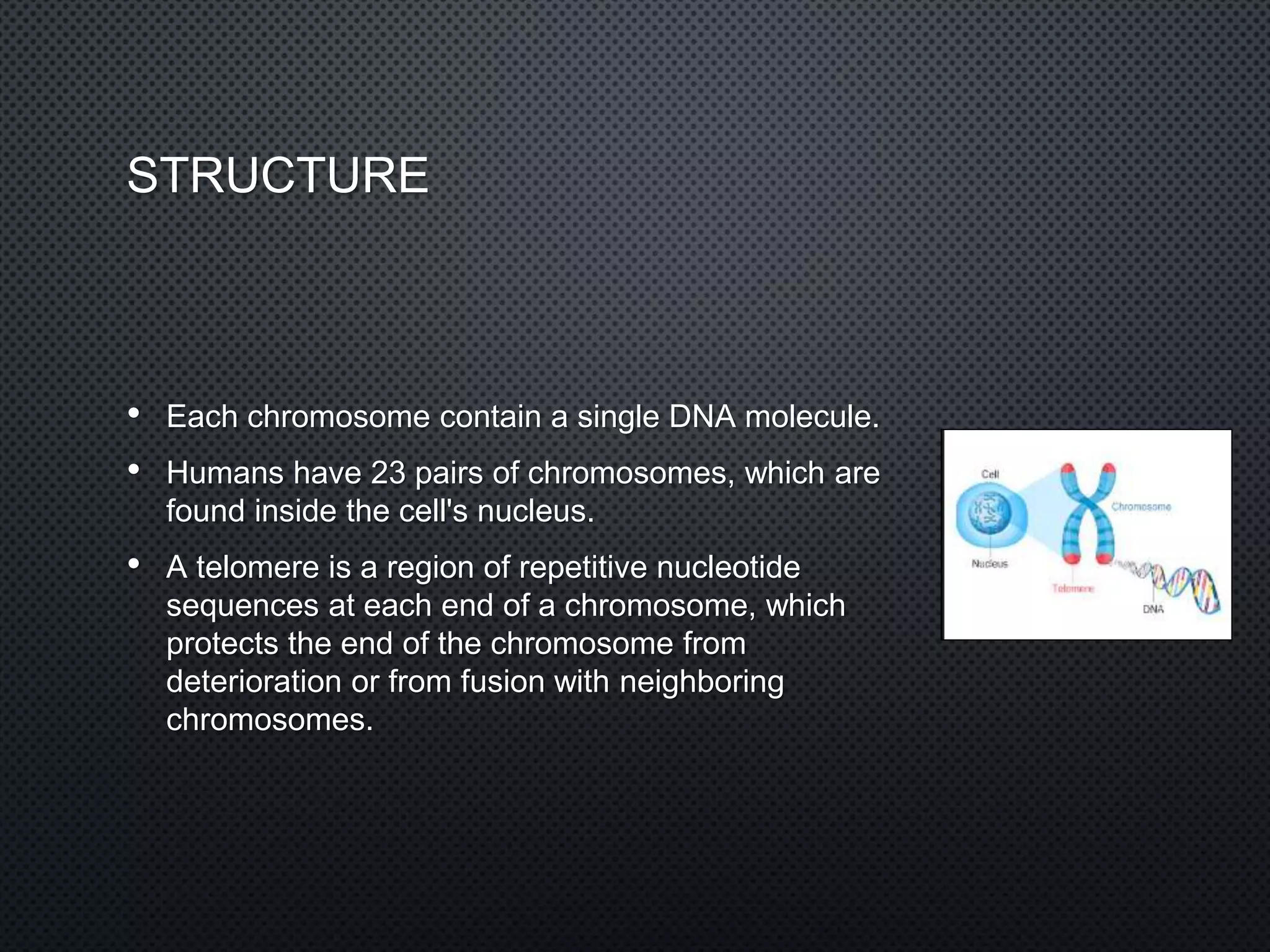
DNA is made up of nucleotides containing phosphate groups, sugars, and nitrogenous bases. The bases on one DNA strand form hydrogen bonds with complementary bases on another strand to form the famous double helix structure. The double helix allows DNA to tightly coil into chromosomes inside cells. Hydrogen bonding between adenine-thymine and guanine-cytosine base pairs gives DNA its stable double-stranded structure.