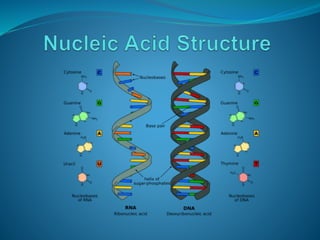
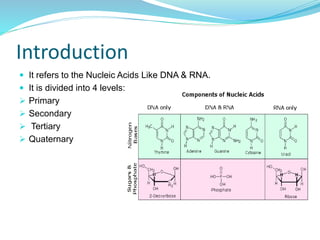
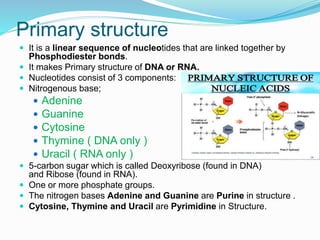
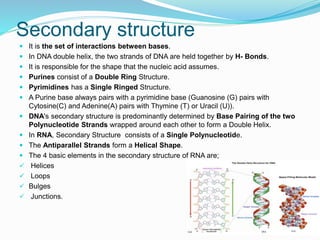
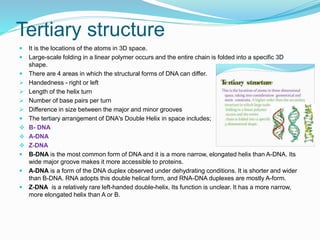
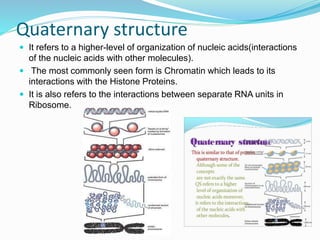
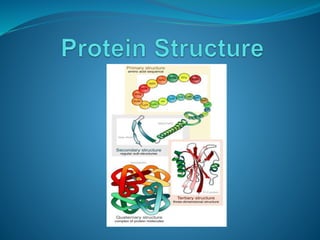
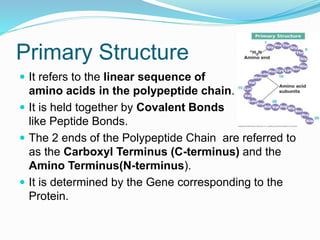
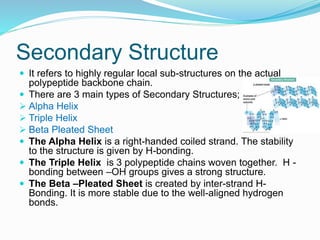
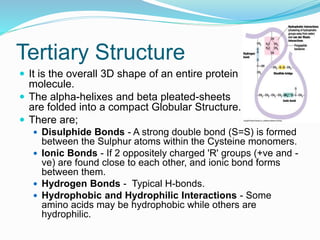
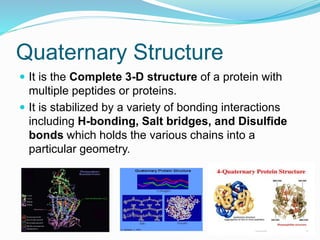
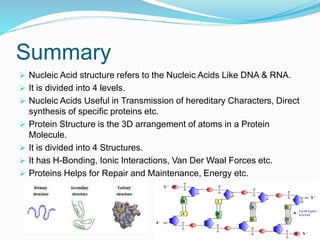
The document discusses the structure and functions of nucleic acids and proteins. It describes the four levels of structure for both - primary, secondary, tertiary, and quaternary. For nucleic acids, this includes the nucleotide components, base pairing, and interactions with other molecules. For proteins, it includes the amino acid sequence, folding patterns like alpha helices and beta sheets, and bonding interactions that give shape. Nucleic acids function to store and transmit genetic information and direct protein synthesis. Proteins function in roles like repair, energy, movement, catalysis, transport, and structure.