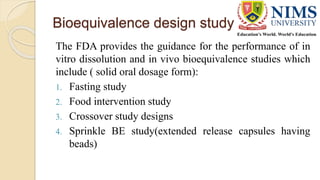
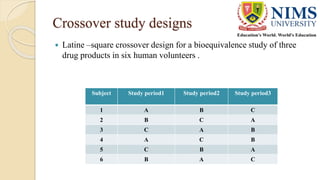
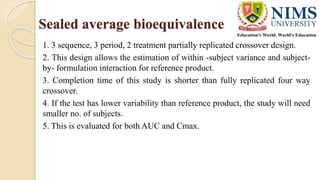
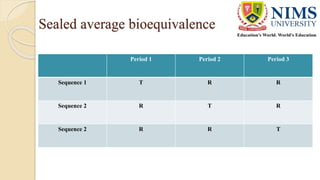
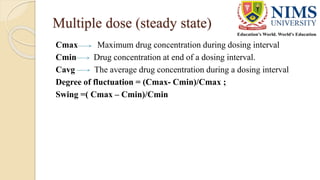
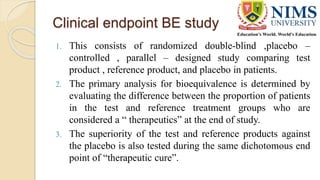
The document discusses the design and requirements of bioequivalence studies, essential in demonstrating that a new drug product performs similarly to an approved one. It outlines types of equivalences (chemical, pharmaceutical, bioequivalence, and therapeutic) and details both in vivo and in vitro study methodologies along with their protocols. Additionally, it highlights the challenges in establishing bioequivalence for highly variable drugs and the importance of adhering to regulatory guidelines during study design.