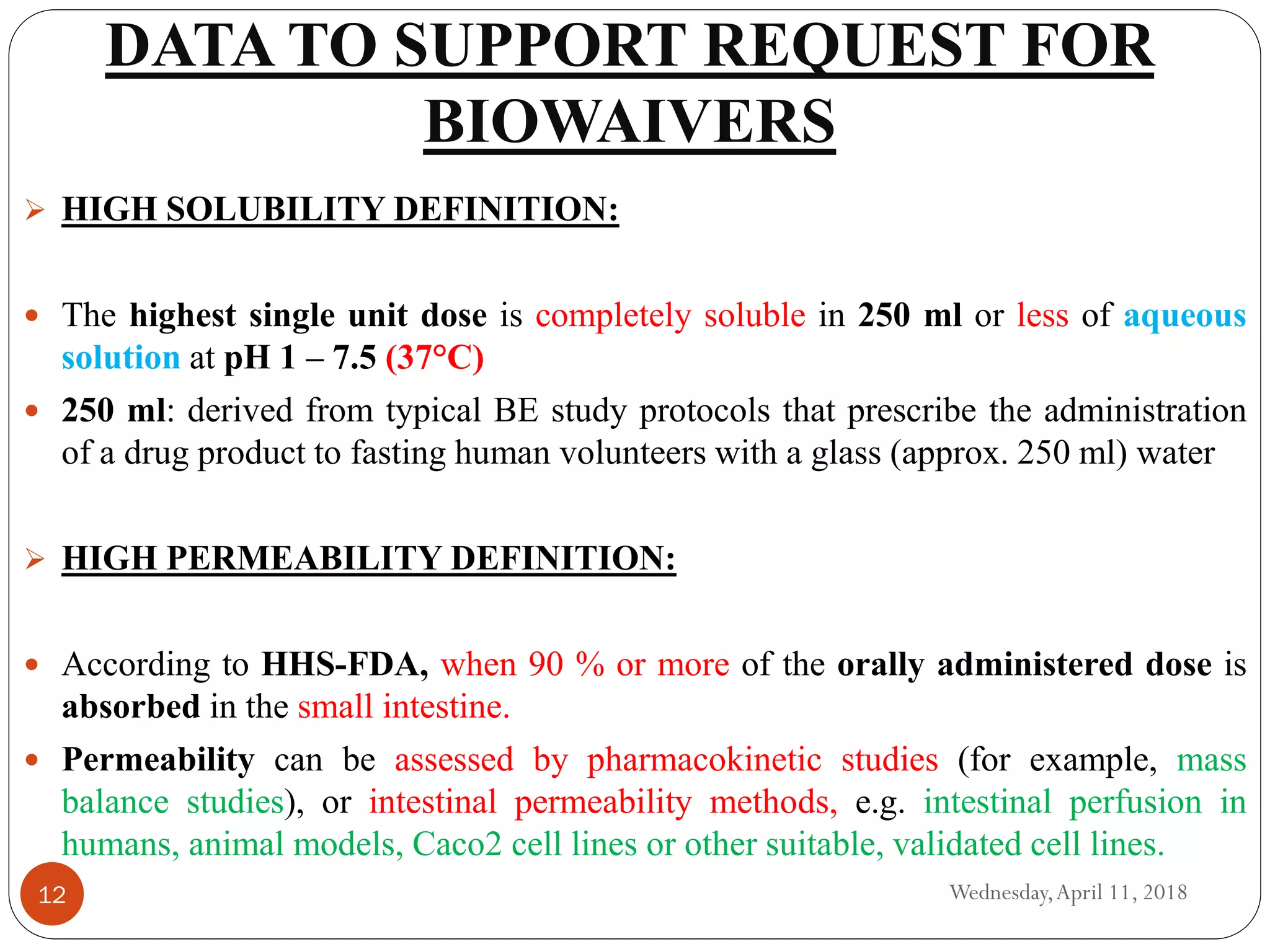
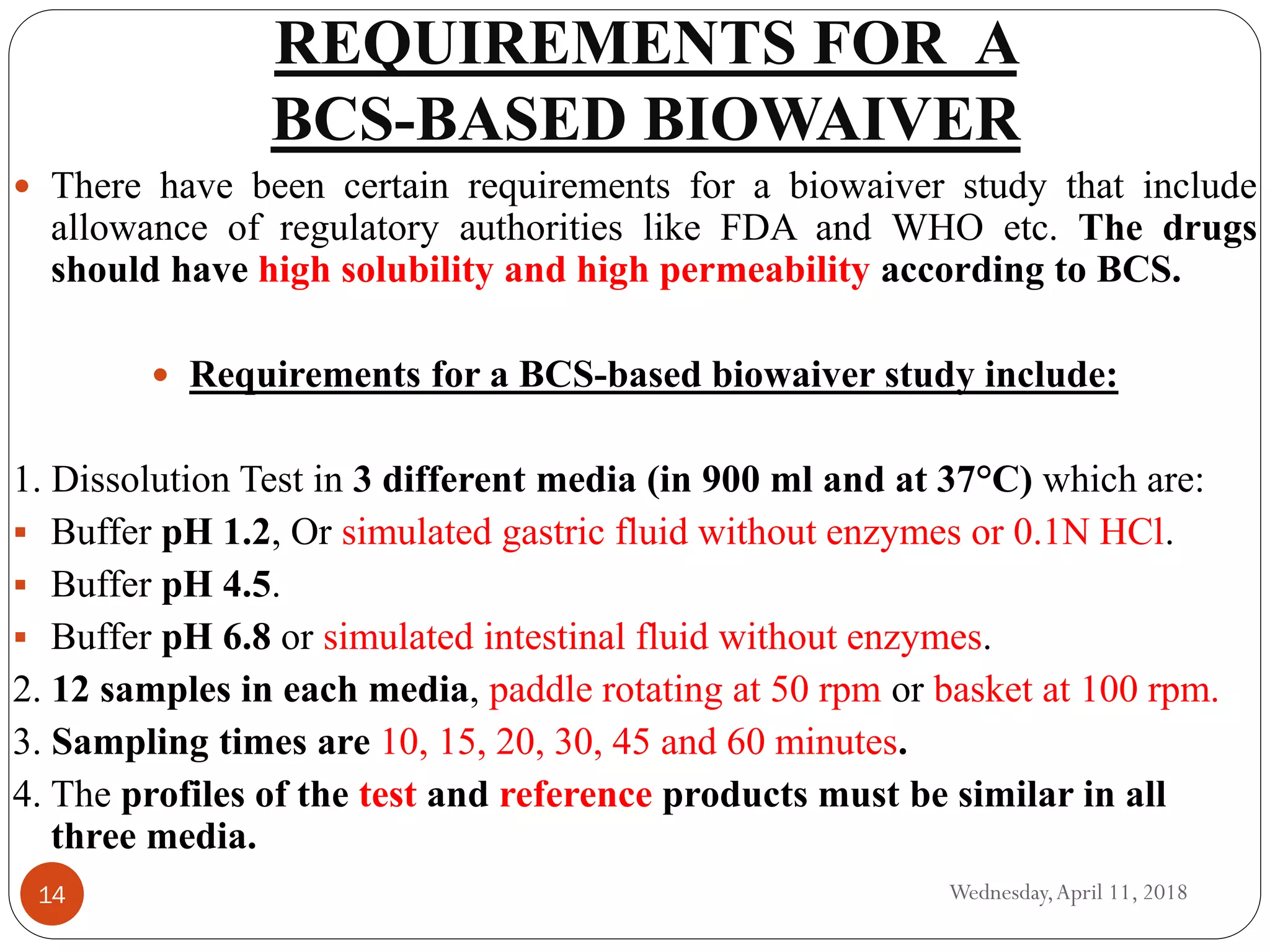
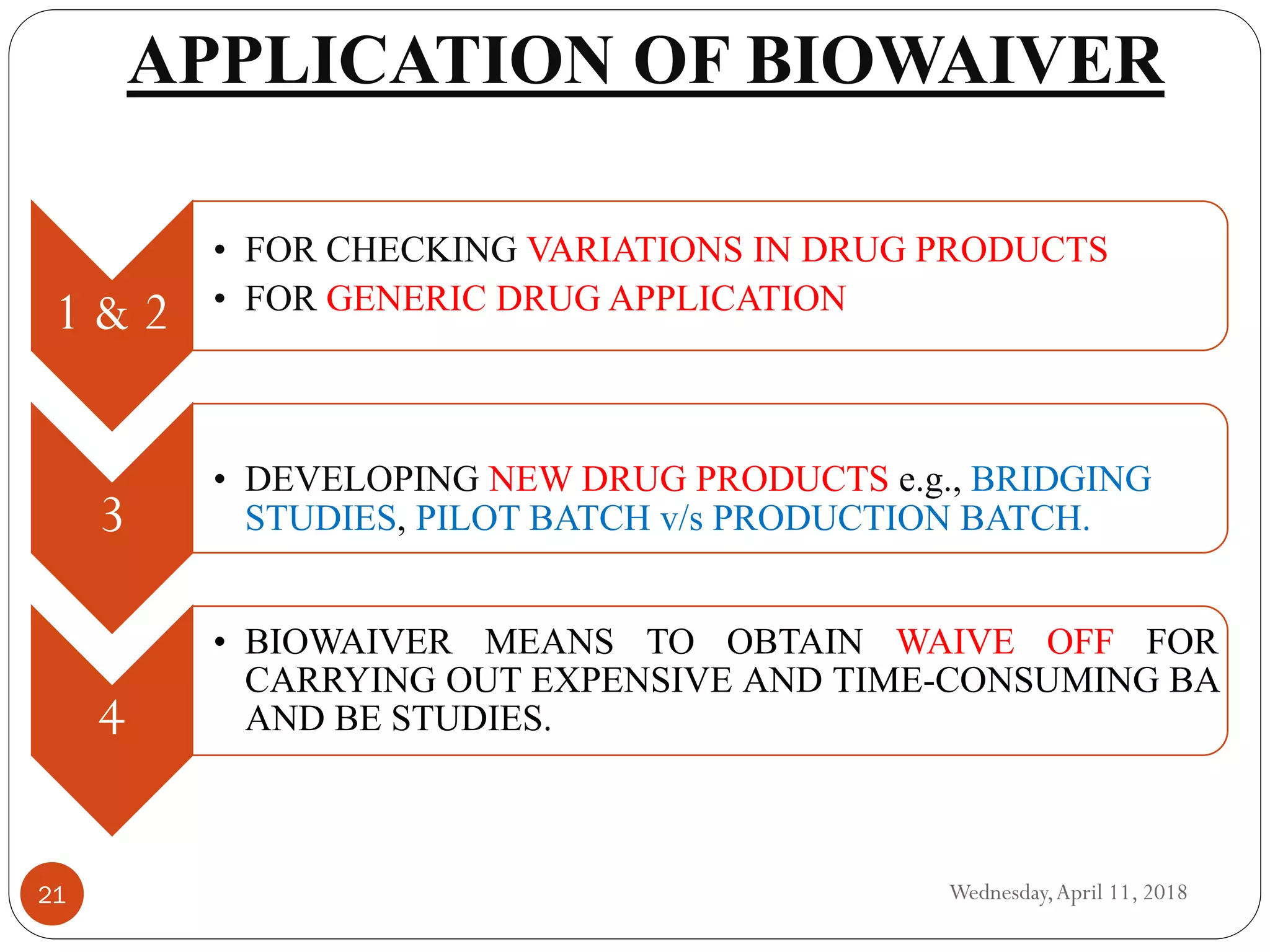
Biowaivers allow for regulatory drug approvals based on in-vitro evidence of equivalence, bypassing the need for in-vivo bioequivalence studies, initiated by the FDA's biopharmaceutics classification system (BCS) in 1995. This procedure simplifies and expedites the approval process for certain orally administered generic products, provided they meet strict solubility and permeability criteria. While advantageous, biowaivers have limitations and are not applicable to narrow therapeutic range drugs or certain dosage forms.