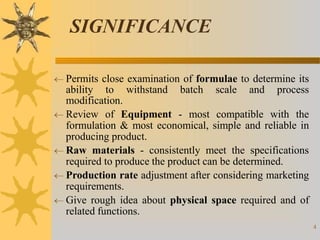
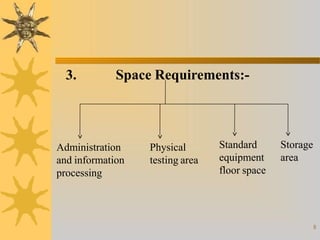
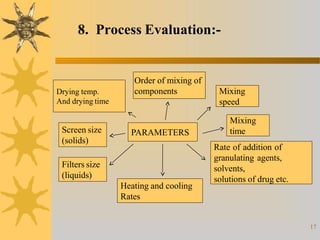
This document discusses techniques for scaling up pilot plant operations in the pharmaceutical industry. It begins with definitions of key terms and explains the significance of pilot plants in permitting examination of formulas at an intermediate scale. The document outlines general considerations for pilot plant operations, including personnel requirements, equipment used, production rates, and process evaluation. It also covers master manufacturing procedures, product stability testing, and GMP compliance. Advantages are given as personnel can observe scale up runs and quality materials can be accessed, while disadvantages include reduced interaction between formulators and production staff.